Pentafluorosulfanyl (SF5) as a Superior 19F Magnetic Resonance Reporter Group: Signal Detection and Biological Activity of Teriflunomide Derivatives
- PMID: 34666481
- PMCID: PMC8630787
- DOI: 10.1021/acssensors.1c01024
Pentafluorosulfanyl (SF5) as a Superior 19F Magnetic Resonance Reporter Group: Signal Detection and Biological Activity of Teriflunomide Derivatives
Abstract
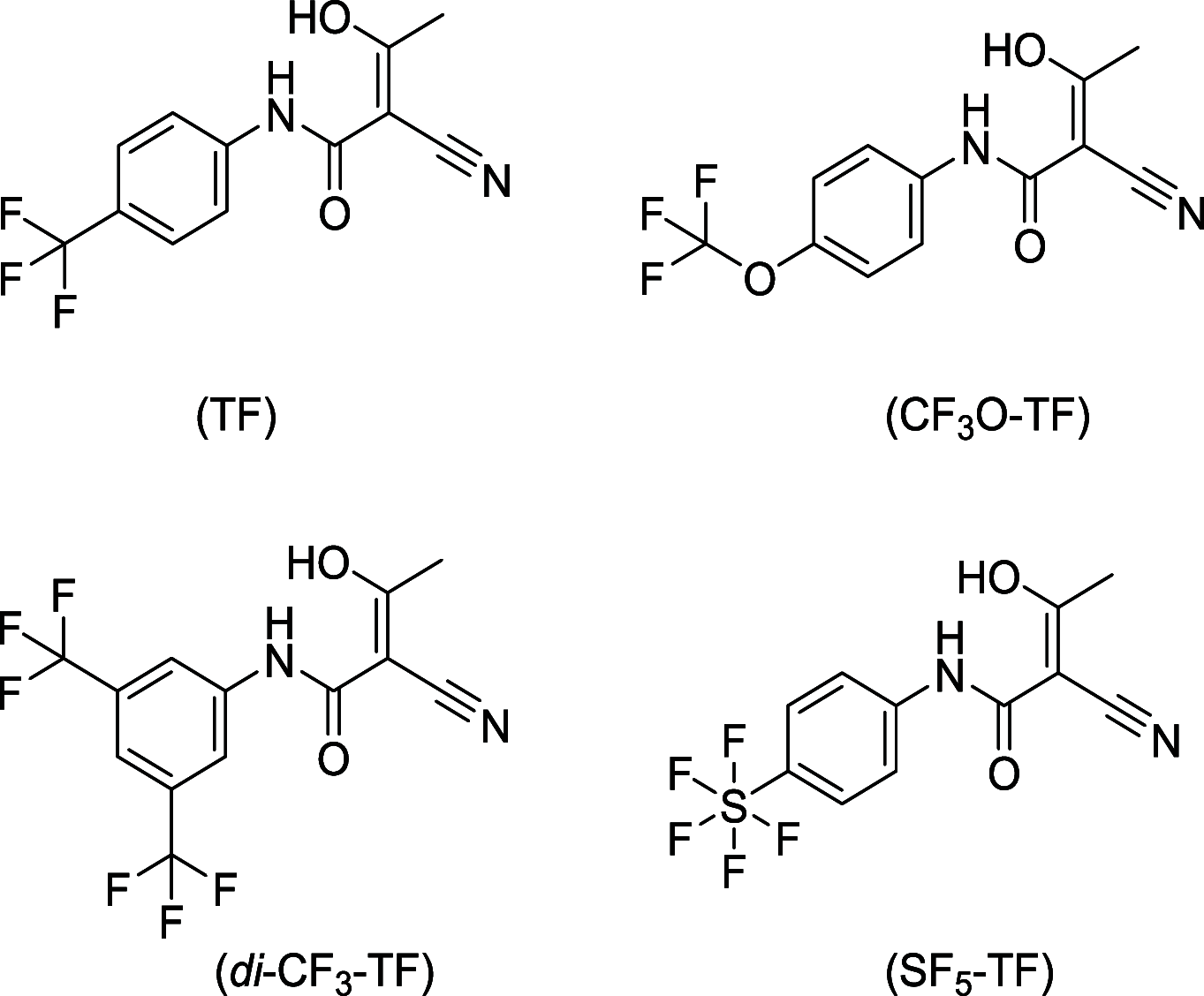
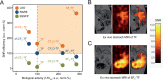
Fluorine (19F) magnetic resonance imaging (MRI) is severely limited by a low signal-to noise ratio (SNR), and tapping it for 19F drug detection in vivo still poses a significant challenge. However, it bears the potential for label-free theranostic imaging. Recently, we detected the fluorinated dihydroorotate dehydrogenase (DHODH) inhibitor teriflunomide (TF) noninvasively in an animal model of multiple sclerosis (MS) using 19F MR spectroscopy (MRS). In the present study, we probed distinct modifications to the CF3 group of TF to improve its SNR. This revealed SF5 as a superior alternative to the CF3 group. The value of the SF5 bioisostere as a 19F MRI reporter group within a biological or pharmacological context is by far underexplored. Here, we compared the biological and pharmacological activities of different TF derivatives and their 19F MR properties (chemical shift and relaxation times). The 19F MR SNR efficiency of three MRI methods revealed that SF5-substituted TF has the highest 19F MR SNR efficiency in combination with an ultrashort echo-time (UTE) MRI method. Chemical modifications did not reduce pharmacological or biological activity as shown in the in vitro dihydroorotate dehydrogenase enzyme and T cell proliferation assays. Instead, SF5-substituted TF showed an improved capacity to inhibit T cell proliferation, indicating better anti-inflammatory activity and its suitability as a viable bioisostere in this context. This study proposes SF5 as a novel superior 19F MR reporter group for the MS drug teriflunomide.
Keywords: DHODH; MRI; MRS; SF5; fluorine; teriflunomide.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Niendorf T.; Ji Y.; Waiczies S., Chapter 11 Fluorinated Natural Compounds and Synthetic Drugs. In Fluorine Magnetic Resonance Imaging, 1 ed.; Ahrens E. T.; Flögel U. Ed. Pan Stanford Publishing: 2016; pp. 311–344, 10.1201/9781315364605-12. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

