COVID-19-related policy changes for methadone take-home dosing: A multistate survey of opioid treatment program leadership
- PMID: 34666636
- PMCID: PMC8810732
- DOI: 10.1080/08897077.2021.1986768
COVID-19-related policy changes for methadone take-home dosing: A multistate survey of opioid treatment program leadership
Abstract
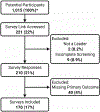
Background: In the United States, methadone for treatment of opioid use disorder is dispensed via highly-regulated accredited opioid treatment programs (OTP). During the COVID-19 pandemic, federal regulations were loosened, allowing for greater use of take-home methadone doses. We sought to understand how OTP leaders responded to these policy changes. Methods: We distributed a multistate electronic survey from September to November 2020 of OTP leadership to members of the American Association for the Treatment of Opioid Dependence (AATOD) who self-identified as leaders of OTPs. We asked study participants about how their OTP(s) implemented COVID-19-related policy changes into their clinical practice focusing on provision of take-home methadone doses, factors used to determine patient stability, and potential concerns about increased take-home doses. We used Chi-square test to compare survey responses between characterizations of the OTPs. Results: Of 170 survey respondents (17% response rate), the majority represented leadership of for-profit OTPs (69%) and were in a Southern state (54%). Routine allowances and practices related to take-home methadone doses varied across OTPs during the COVID-19 pandemic: 80 (47%) reported 14 days for newly enrolled patients (within past 90 days), 89 (52%) reported 14 days for "less stable" patients, and 112 (66%) reported 28 days for "stable" patients. Conclusions: We found that not all eligible OTP leaders adopted the practice of routinely allowing newly enrolled, "less stable," and "stable" patients on methadone to have increased take-home doses up to the limit allowed by federal regulations during COVID-19. The pandemic provides an opportunity to critically re-evaluate long-established methadone and OTP regulations in preparation for future emergencies.
Keywords: COVID-19; Methadone; opioid treatment programs; opioid-related disorders; survey.
Conflict of interest statement
Disclosure statement
KBS serves on the Boards of Directors of the American Association for the Treatment of Opioid Dependence, and the Maryland Association for the Treatment of Opioid Dependence. He also serves on the Center for Substance Abuse Treatment National Advisory Council of the U.S. Department of Health and Human Services, Substance Abuse and Mental Health Administration. The other authors – XAL, JDP, PTK, and GC – declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. No potential conflict of interest was reported by the author(s).
Figures

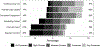
References
-
- Substance Abuse and Mental Health Services Administration. Opioid treatment program (OTP) guidance. Substance Abuse and Mental Health Services Administration; 2020. https://www.samhsa.gov/sites/default/files/otp-guidance-20200316.pdf.
-
- Samet JH, Botticelli M, Bharel M. Methadone in primary care – one small step for congress, one giant leap for addiction treatment. N Engl J Med. 2018;379(1):7–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

