Neoadjuvant and Adjuvant Nivolumab and Lirilumab in Patients with Recurrent, Resectable Squamous Cell Carcinoma of the Head and Neck
- PMID: 34667025
- PMCID: PMC9401515
- DOI: 10.1158/1078-0432.CCR-21-2635
Neoadjuvant and Adjuvant Nivolumab and Lirilumab in Patients with Recurrent, Resectable Squamous Cell Carcinoma of the Head and Neck
Abstract
Purpose: Surgery often represents the best chance for disease control in locoregionally recurrent squamous cell carcinoma of the head and neck (SCCHN). We investigated dual immune-checkpoint inhibition [anti-PD-1, nivolumab (N), and anti-KIR, lirilumab (L)] before and after salvage surgery to improve disease-free survival (DFS).
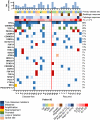
Patients and methods: In this phase II study, patients received N (240 mg) + L (240 mg) 7 to 21 days before surgery, followed by six cycles of adjuvant N + L. Primary endpoint was 1-year DFS; secondary endpoints were safety, pre-op radiologic response, and overall survival (OS). Correlatives included tumor sequencing, PD-L1 scoring, and immunoprofiling.
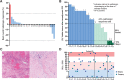
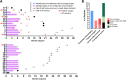
Results: Among 28 patients, the median age was 66, 86% were smokers; primary site: 9 oral cavity, 9 oropharynx, and 10 larynx/hypopharynx; 96% had prior radiation. There were no delays to surgery. Grade 3+ adverse events: 11%. At the time of surgery, 96% had stable disease radiologically, one had progression. Pathologic response to N + L was observed in 43% (12/28): 4/28 (14%) major (tumor viability, TV ≤ 10%) and 8/28 (29%) partial (TV ≤ 50%). PD-L1 combined positive score (CPS) at surgery was similar regardless of pathologic response (P = 0.71). Thirteen (46%) recurred (loco-regional = 10, distant = 3). Five of 28 (18%) had positive margins, 4 later recurred. At median follow-up of 22.8 months, 1-year DFS was 55.2% (95% CI, 34.8-71.7) and 1-year OS was 85.7% (95% CI, 66.3-94.4). Two-year DFS and OS were 64% and 80% among pathologic responders.
Conclusions: (Neo)adjuvant N + L was well tolerated, with a 43% pathologic response rate. We observed favorable DFS and excellent 2-year OS among high-risk, previously treated patients exhibiting a pathologic response. Further evaluation of this strategy is warranted.See related commentary by Sacco and Cohen, p. 435.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
-
Inevitable Progress-Relying on the Immune System, Not Chance.Clin Cancer Res. 2022 Feb 1;28(3):435-437. doi: 10.1158/1078-0432.CCR-21-3739. Epub 2021 Nov 30. Clin Cancer Res. 2022. PMID: 34848533
References
-
- Bourhis J, Le Maitre A, Baujat B, Audry H, Pignon JP. Meta-analysis of chemotherapy in head, neck cancer collaborative group, meta-analysis of radiotherapy in carcinoma of head, neck collaborative group, meta-analysis of chemotherapy in nasopharynx carcinoma collaborative group individual patients' data meta-analyses in head and neck cancer. Curr Opin Oncol 2007;19:188. - PubMed
-
- Brockstein B, Haraf DJ, Rademaker AW, Kies MS, Stenson KM, Rosen F, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol 2004;15:1179. - PubMed
-
- Ho AS, Kraus DH, Ganly I, Lee NY, Shah JP, Morris LG. Decision making in the management of recurrent head and neck cancer. Head Neck 2014;36:144–51. - PubMed
-
- Agra IM, Carvalho AL, Pontes E, Campos OD, Ulbrich FS, Magrin J, et al. Postoperative complications after en bloc salvage surgery for head and neck cancer. Arch Otolaryngol Head Neck Surg 2003;129:1317. - PubMed
-
- Goodwin WJ Jr. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope 2000;110:1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

