Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes
- PMID: 34667060
- PMCID: PMC8719459
- DOI: 10.1183/13993003.02730-2021
Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes
Abstract
The GINA Strategy Report provides clinicians with an annually updated evidence-based strategy for asthma management and prevention. This article summarizes key recommendations from GINA 2021, and the evidence underpinning the new changes.
Conflict of interest statement
Conflict of interest: L.B. Bacharier reports receiving royalties for textbooks from Elsevier; consulting fees from GlaxoSmithKline, Teva, AstraZeneca, Sanofi, Regeneron, Vectura, Circassia, Kinaset, Merck and Boehringer Ingelheim; honoraria for lectures from GlaxoSmithKline, Genentech/Novartis, Teva Honoraria, AstraZeneca, Sanofi/Regeneron and WebMD/Medscape; participation on a data safety monitoring board or advisory board for DBV Technologies, AstraZeneca and Vertex (CF Foundation); leadership or fiduciary roles in other board, society, committee or advocacy group, paid or unpaid for AAAAI Board of Directors (unpaid) and ABAI Board of Directors, in the past 36 months. E.D. Bateman reports receiving advisory board, consultant and speaker fees from AstraZeneca, Sanofi Genzyme, Regeneron, Novartis and ALK; speaker fees from Boehringer Ingelheim, Menarini and Orion; and advisory board and speaker fees from Chiesi, since the initial planning of this work; E.D. Bateman reports receiving consulting fees from AstraZeneca, Sanofi Genzyme, Regeneron, Novartis and ALK; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Menarini, Orion, Novartis, Regeneron, Sanofi Genzyme, Chiesi and ALK; support for attending meetings and/or travel from Novartis (to attend European Respiratory Society Congress 2021); participation on a data safety monitoring board or advisory board from AstraZeneca, ALK, Sanofi Genzyme, Regeneron, Novartis and Chiesi; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for Global Initiative for Asthma (Member of Board and Science Committee, unpaid), in the past 36 months. L-P. Boulet reports grants paid to institution for multicentre or investigator-generated clinical studies from Amgen, AstraZeneca, GlaxoSmithKline, Merck, Novartis and Sanofi-Regeneron; royalties from UpToDate; consultancy fees from AstraZeneca, Novartis, GlaxoSmithKline, Merck and Sanofi-Regeneron; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Covis, GlaxoSmithKline, Novartis, Merck and Sanofi; and is Chair of the Global Initiative for Asthma (GINA) Board of Directors, President of the Global Asthma Organisation (Interasma), member of the Canadian Thoracic Society Respiratory Guidelines Committee, and Laval University Chair on Knowledge Transfer, Prevention and Education in Respiratory and Cardiovascular Health. C.E. Brightling reports receiving grants or contracts from GSK, AZ, Novartis, Chiesi, BI, Mologic, 4DPharma, Gossamer, Merck and Roche/Genentech (all paid to institution); consulting fees from AZ, Novartis, Chiesi, BI, Mologic, TEVA, 4DPharma, Gossamer, Regeneron, Sanofi and Roche/Genentech (all paid to institution), in the past 36 months. G.G. Brusselle reports receiving payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Sanofi and Teva; participation on a data safety monitoring board or advisory board for AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, MSD, Novartis, Sanofi and Teva, in the past 36 months. R. Buhl reports grants from Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Roche; lecture honoraria from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Novartis, Roche, Sanofi, Teva; participation on advisory board from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Roche, Sanofi, Teva; outside the submitted work; and has acted as a Member of the Scientific Committee of the Global Initiative for Asthma (GINA). A.A. Cruz reports lecture honoraria from Abdi Ibrahim, AstraZeneca, Boehringer Ingelheim, Chiesi, Glennmark, GSK, Mylan, Novartis and Sanofi; travel support from AstraZeneca, Boehringer Ingelheim and Chiesi, outside the submitted work. L. Duijts reports grants from European Union's Horizon 2020 research and innovation programme (LIFECYCLE, grant agreement number 733206, 2016; EUCAN-Connect grant agreement number 824989; ATHLETE, grant agreement number 874583), outside the submitted work. J.M. FitzGerald reports grants from AstraZeneca, GSK, TEVA, Sanofi, CIHR and NIH; consulting fees and lecture honoraria from AstraZeneca, GSK, TEVA and Sanofi; outside the submitted work. L.J. Fleming reports consulting fees from GSK, Sanofi, Respiri UK and AstraZeneca; lecture honoraria from Novartis and Teva, outside the submitted work. H. Inoue reports grants to institution from Boehringer Ingelheim, GlaxoSmithKline, Kyorin, MerckSharp&Dohme and Ono; personal consulting fees from Boehringer Ingelheim; lecture honoraria from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Kyorin, MerckSharp&Dohme, Novartis and Sanofi; has participated on an advisory board for AstraZeneca, GlaxoSmithKline, Novartis and Sanofi; board leadership with Japanese Respiratory Society (Board of Directors and Chairman of Education Committee) and Japanese Society of Allergology (Former Chairman of Education Committee), outside the submitted work. F.W. Ko reports support for travel from Novartis, Boehringer Ingelheim and GSK, outside the submitted work. J.A. Krishnan reports grants from Regeneron Pharmaceuticals, Sergey Brin Family Foundation, Patient Centered Outcomes Research Institute, COPD Foundation and American Lung Association; consulting fees from GlaxoSmithKline for COVID-19 mAb; board leadership at COPD Foundation and Respiratory Health Association, outside the submitted work. M.L. Levy reports grants from Consorzio Futuro in Ricerca and AstraZeneca; royalties from Class Publishing and Taylor & Francis; consulting fees from Respiri, Ltd, TEVA and NSHI Ltd; lecture honoraria from TEVA, Novartis, Chiesi and Orion; payment for expert testimony from HM Coroner Walthamstow, London, and HM Coroner, Waltham Forrest, London; travel support from Orion Pharmaceuticals, Menarini Pharmaceuticals, Napp Pharmaceuticals and TEVA; participation on advisory board at Chiesi, Novartis, GSK and Sandoz; and board leadership at GINA (Board of Directors), outside the submitted work. K. Mortimer has participated as member of advisory boards for AstraZeneca, outside the submitted work. P.M. Pitrez reports consulting fees and lecture honoraria from AstraZeneca, Boehringer Ingelheim, GSK, Sanofi and Novartis; travel support from GSK and Boehringer Ingelheim; and held an unpaid position as President of the Brazilian Severe Asthma Group, outside the submitted work. H.K. Reddel reports grants from AstraZeneca, GlaxoSmithKline and Novartis; consulting fees from Novartis; lecture payments from AstraZeneca, GlaxoSmithKline, Teva, Boehringer Ingelheim, Sanofi and Chiesi; participation on advisory boards for AstraZeneca, GlaxoSmithKline, Novartis, Chiesi and Sanofi; acted as Chair of Scientific Committee and Member of Board of Directors for Global Initiative for Asthma, and acted as Member of Australian Asthma Guidelines Committee for National Asthma Council, outside the submitted work. A. Sheikh reports grants from HDRUK BREATHE Hub, and Asthma UK Centre for Applied Research; and participated in the AstraZeneca Unmet Asthma Need Working Group; outside the submitted work. A.A. Yorgancioglu reports grants from MSD, AstraZeneca, Sanofi and Novartis; consulting fees from AstraZeneca, GSK, Novartis, Sanofi, Chiesi and Deva; lecture honoraria from AstraZeneca, Abdi İbrahim, GSK, Novartis, Sandoz, Chiesi and Vem İlaç; advisory board participation from GSK and AstraZeneca; outside the submitted work. All other authors have nothing to disclose.
Figures

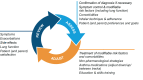


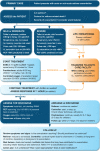

References
-
- Global Asthma Network . The Global Asthma Report 2018. Auckland, Global Asthma Network, 2018. http://globalasthmareport.org (Last accessed Sept 2021).
-
- National Heart, Lung, and Blood Institute National Asthma Education and Prevention Program; World Health Organization . Global Initiative for Asthma . Bethesda, National Institutes of Health; 1995. National Institutes of Health Publication number 95-3659.
-
- Global Initiative for Asthma . Methodology. Fontana, Global Initiative for Asthma, 2021. https://ginasthma.org/about-us/methodology (Last accessed Sept 2021).
-
- Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention. Fontana, Global Initiative for Asthma, 2021. www.ginasthma.org/reports (Last accessed Sept 2021).
