Overcoming Gemcitabine Resistance in Pancreatic Cancer Using the BCL-XL-Specific Degrader DT2216
- PMID: 34667112
- PMCID: PMC8742767
- DOI: 10.1158/1535-7163.MCT-21-0474
Overcoming Gemcitabine Resistance in Pancreatic Cancer Using the BCL-XL-Specific Degrader DT2216
Abstract
Pancreatic cancer is the third most common cause of cancer-related deaths in the United States. Although gemcitabine is the standard of care for most patients with pancreatic cancer, its efficacy is limited by the development of resistance. This resistance may be attributable to the evasion of apoptosis caused by the overexpression of BCL-2 family antiapoptotic proteins. In this study, we investigated the role of BCL-XL in gemcitabine resistance to identify a combination therapy to more effectively treat pancreatic cancer. We used CRISPR-Cas9 screening to identify the key genes involved in gemcitabine resistance in pancreatic cancer. Pancreatic cancer cell dependencies on different BCL-2 family proteins and the efficacy of the combination of gemcitabine and DT2216 (a BCL-XL proteolysis targeting chimera or PROTAC) were determined by MTS, Annexin-V/PI, colony formation, and 3D tumor spheroid assays. The therapeutic efficacy of the combination was investigated in several patient-derived xenograft (PDX) mouse models of pancreatic cancer. We identified BCL-XL as a key mediator of gemcitabine resistance. The combination of gemcitabine and DT2216 synergistically induced cell death in multiple pancreatic cancer cell lines in vitro In vivo, the combination significantly inhibited tumor growth and prolonged the survival of tumor-bearing mice compared with the individual agents in pancreatic cancer PDX models. Their synergistic antitumor activity is attributable to DT2216-induced degradation of BCL-XL and concomitant suppression of MCL-1 by gemcitabine. Our results suggest that DT2216-mediated BCL-XL degradation augments the antitumor activity of gemcitabine and their combination could be more effective for pancreatic cancer treatment.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures

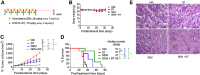
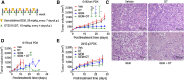
![Figure 2. The combination of DT2216 and gemcitabine synergistically kills pancreatic cancer cells in vitro. A, Percentage of total apoptotic cells (the sum of early [Annexin V+] and late [PI+/Annexin V+] apoptotic cells) after the cells were treated with vehicle (Veh) or with the indicated concentrations of DT2216 (DT) or gemcitabine (GEM) alone or in combination for 72 hours. Data are presented as the mean ± SD of four independent experiments for each cell line and condition. a, b and c, P < 0.05 versus. Veh, DT and GEM, respectively. B, Percentage of total apoptotic G-68 primary pancreatic cancer cells after they were treated with vehicle (Veh) or the indicated concentrations of DT or gemcitabine alone or in combinations for 72 hours. Data are presented as mean ± SD (n = 3 independent experiments). *, P < 0.05; ****, P < 0.0001. Representative flow cytometric quadruple graphs for A and B are shown in Supplementary Fig. S2A and S2B, respectively. C, Percentage viability of G-68 cells after they were treated with increasing concentrations of DT, gemcitabine, or their combination (GEM+DT; 1:1 ratio) for 72 hours. The table shows the IC50 values of DT, gemcitabine, or their combination (GEM+DT). IC50 values are shown for a representative experiment out of three independent experiments. The CI value (< 1) indicates a synergy between DT and gemcitabine. D, G-68 cells were treated with the indicated concentrations of DT or gemcitabine alone or in combination for 72 hours followed by incubation in drug-free medium for another two weeks. Crystal violet staining was performed to visualize the colonies. Data are representative images from two independent experiments. E, G-68 cells were seeded in a 48-well plate and allowed 48 hours for spheroid formation before DT and/or gemcitabine treatment. Micrographs (magnification at 50×) of spheroids in the corresponding wells after they were treated with the indicated concentrations of DT or gemcitabine alone or in combination for 10 days are shown. F, Microphotographs (magnification 50×) of spheroids from the marked areas in E. G, Quantification of the number of spheroids from E as a percentage of Veh. a, b and c, P < 0.05 versus Veh, DT, and GEM, respectively. Data presented in E–G are representative of three independent experiments. Statistical significance in A, B, and G was determined by two-way ANOVA with Tukey post hoc test.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/036f/9398150/f861e586e7cb/184fig2.gif)

References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15:1028–61. - PubMed
-
- Schniewind B, Christgen M, Kurdow R, Haye S, Kremer B, Kalthoff H, et al. Resistance of pancreatic cancer to gemcitabine treatment is dependent on mitochondria-mediated apoptosis. Int J Cancer 2004;109:182–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

