Loss of Numb promotes hepatic progenitor expansion and intrahepatic cholangiocarcinoma by enhancing Notch signaling
- PMID: 34667161
- PMCID: PMC8526591
- DOI: 10.1038/s41419-021-04263-w
Loss of Numb promotes hepatic progenitor expansion and intrahepatic cholangiocarcinoma by enhancing Notch signaling
Abstract
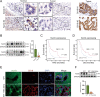
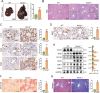
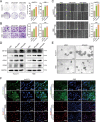
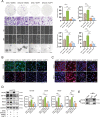
Numb, a stem cell fate determinant, acts as a tumor suppressor and is closely related to a wide variety of malignancies. Intrahepatic cholangiocarcinoma (iCCA) originates from hepatic progenitors (HPCs); however, the role of Numb in HPC malignant transformation and iCCA development is still unclear. A retrospective cohort study indicated that Numb was frequently decreased in tumor tissues and suggests poor prognosis in iCCA patients. Consistently, in a chemically induced iCCA mouse model, Numb was downregulated in tumor cells compared to normal cholangiocytes. In diet-induced chronic liver injury mouse models, Numb ablation significantly promoted histological impairment, HPC expansion, and tumorigenesis. Similarly, Numb silencing in cultured iCCA cells enhanced cell spheroid growth, invasion, metastasis, and the expression of stem cell markers. Mechanistically, Numb was found to bind to the Notch intracellular domain (NICD), and Numb ablation promoted Notch signaling; this effect was reversed when Notch signaling was blocked by γ-secretase inhibitor treatment. Our results suggested that loss of Numb plays an important role in promoting HPC expansion, HPC malignant transformation, and, ultimately, iCCA development in chronically injured livers. Therapies targeting suppressed Numb are promising for the treatment of iCCA.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

