Boride-derived oxygen-evolution catalysts
- PMID: 34667176
- PMCID: PMC8526748
- DOI: 10.1038/s41467-021-26307-7
Boride-derived oxygen-evolution catalysts
Abstract
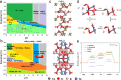
Metal borides/borates have been considered promising as oxygen evolution reaction catalysts; however, to date, there is a dearth of evidence of long-term stability at practical current densities. Here we report a phase composition modulation approach to fabricate effective borides/borates-based catalysts. We find that metal borides in-situ formed metal borates are responsible for their high activity. This knowledge prompts us to synthesize NiFe-Boride, and to use it as a templating precursor to form an active NiFe-Borate catalyst. This boride-derived oxide catalyzes oxygen evolution with an overpotential of 167 mV at 10 mA/cm2 in 1 M KOH electrolyte and requires a record-low overpotential of 460 mV to maintain water splitting performance for over 400 h at current density of 1 A/cm2. We couple the catalyst with CO reduction in an alkaline membrane electrode assembly electrolyser, reporting stable C2H4 electrosynthesis at current density 200 mA/cm2 for over 80 h.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Li H, et al. Earth‐abundant iron diboride (FeB2) nanoparticles as highly active bifunctional electrocatalysts for overall water splitting. Adv. Energy Mater. 2017;7:1700513. doi: 10.1002/aenm.201700513. - DOI
LinkOut - more resources
Full Text Sources

