Performance of the LumiraDx Microfluidic Immunofluorescence Point-of-Care SARS-CoV-2 Antigen Test in Asymptomatic Adults and Children
- PMID: 34668536
- PMCID: PMC8973256
- DOI: 10.1093/ajcp/aqab173
Performance of the LumiraDx Microfluidic Immunofluorescence Point-of-Care SARS-CoV-2 Antigen Test in Asymptomatic Adults and Children
Abstract
Objectives: The LumiraDx SARS-CoV-2 Ag Test has previously been shown to accurately detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals symptomatic for coronavirus disease 2019 (COVID-19). This evaluation investigated the LumiraDx SARS-CoV-2 Ag Test as an aid in the diagnosis of SARS-CoV-2 infection in asymptomatic adults and children.
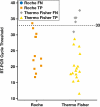
Methods: Asymptomatic individuals at high risk of COVID-19 infection were recruited in 5 point-of-care (POC) settings. Two paired anterior nasal swabs were collected from each participant, tested by using the LumiraDx SARS-CoV-2 Ag Test at the POC, and compared with results from reverse transcription-polymerase chain reaction (RT-PCR) assays (cobas 6800 [Roche Diagnostics] or TaqPath [Thermo Fisher Scientific]). We calculated positive percent agreement (PPA) and negative percent agreement (NPA), then stratified results on the basis of RT-PCR reference platform and cycle threshold.
Results: Of the 222 included study participants confirmed to be symptom-free for at least 2 weeks before testing, the PPA was 82.1% (95% confidence interval [CI], 64.4%-92.1%). The LumiraDx SARS-CoV-2 Ag Test correctly identified 95.8% (95% CI, 79.8%-99.3%) of the samples confirmed positive in fewer than 33 RT-PCR cycles and 100% (95% CI, 85.1%-100%) in fewer than 30 RT-PCR cycles while maintaining 100% NPA.
Conclusions: This rapid, high-sensitivity test can be used to screen asymptomatic patients for acute SARS-CoV-2 infection in clinic- and community-based settings.
Keywords: Asymptomatic; COVID-19; LumiraDx antigen test; RT-PCR; SARS-CoV-2; Sensitivity.
© American Society for Clinical Pathology, 2021.
Figures

References
-
- Rettner R. Coronavirus outbreak officially declared a pandemic, WHO says. Live Science web site. https://www.livescience.com/coronavirus-pandemic-who.html. Published March 11, 2020. Accessed April 25, 2021.
-
- Global Research Collaboration for Infectious Disease Preparedness, World Health Organization. COVID-19 Public Health Emergency of International Concern (PHEIC). Global research and innovation forum: towards a research roadmap. https://www.who.int/publications/m/item/covid-19-public-health-emergency.... Published February 12, 2020. Accessed May 2, 2021.
-
- WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020. World Health Organization Web site. https://www.who.int/director-general/speeches/detail/who-director-genera.... Published March 11, 2020. Accessed May 2, 2021.
-
- Secretary of Health and Human Services. Determination of a public health emergency and declaration that circumstances exist justifying authorizations pursuant to section 564(b) of the Federal Food, Drug, and Cosmetic Act, 210 U.S.C. § 360bbb-3. US Food and Drug Administration web site. https://www.fda.gov/media/135010/download. Published February 4, 2020. Accessed May 2, 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

