First-line nab-paclitaxel plus carboplatin for patients with advanced non-small cell lung cancer: Results of the NEPTUN study
- PMID: 34668662
- PMCID: PMC8607256
- DOI: 10.1002/cam4.4310
First-line nab-paclitaxel plus carboplatin for patients with advanced non-small cell lung cancer: Results of the NEPTUN study
Abstract
Background: Platinum-based chemotherapy remains a first-line standard of care for approximately 30% of patients with non-small cell lung cancer (NSCLC) not harboring a druggable alteration. Favorable efficacy and safety of the nab-paclitaxel/carboplatin (nab-P/C) combination was shown in the pivotal phase 3 trial. However, information on effectiveness of nab-P/C in a real-world setting in Germany is missing. The NEPTUN study prospectively investigated the effectiveness and safety of nab-P/C in patients with advanced NSCLC in a real-world setting.
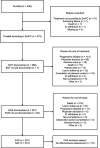
Methods: Patients with advanced or metastatic NSCLC received first-line nab-P/C according to clinical routine. The primary endpoint was 6-month progression-free survival rate (PFS6). Other endpoints included further effectiveness parameters, safety and quality of life. Data were analyzed descriptively.
Results: 408 patients were enrolled. PFS6 was 40.8% (95% confidence interval [CI], 35.3-46.2); median PFS was 5.2 months (95% CI, 4.5-5.7). overall response rate was 41.5% (95% CI, 36.3-46.8). Median overall survival (OS) was 10.5 months (95% CI, 9.2-11.6). Subgroup analyses revealed median OS for squamous versus non-squamous histology (11.8 months [95% CI, 9.2-13.8] vs. 9.6 months [95% CI, 7.7-11.2]) and age ≥70 versus <70 years (11.7 months [95% CI, 9.4-14.3] vs. 9.6 months [95% CI, 7.5-11.2]). Most common treatment-emergent adverse events (TEAEs) were anemia (26.5%), leukopenia (25.7%), and thrombocytopenia (16.6%). Mostly reported grade 3/4 TEAEs were leukopenia (10.2%), anemia (8.6%), and pneumonia (5.1%). nab-paclitaxel-related deaths as reported by the investigator occurred in 0.8% of patients.
Conclusion: These real-world data support the effectiveness and safety of nab-P/C as first-line treatment for patients with advanced NSCLC independent of tumor histology. The results are comparable with the pivotal phase 3 trial. No new safety signals emerged.
Keywords: Germany; carboplatin; nab-paclitaxel; non-small cell lung carcinoma (NSCLC); real-world.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
S. Budweiser, M. Chiabudini, T. Dechow, L. Fischer von Weikersthal, A. Nacke, K. Potthoff, J. Riera‐Knorrenschild, H. Schulz, D. Täuscher, and M. Welslau declare no conflict of interest concerning the topic of this publication. Outside of the submitted work, B. Hackanson received personal fees from Boehringer Inghelheim, MSD, BMS, and Pfizer; J. Janssen received personal fees from Amgen/Onyx, Baxter, Bayer, Biotest, BMS, Celgene, Janssen‐Cilag Ltd, Kedrion Biopharma, Eli Lilly, Merck Serono, Novartis, Octapharma, Pfizer, Pharma Mar, Puma Biotechnology, Roche, Sanofi and Teva Pharmaceutical, Abbvie, Ipsen, and Servier.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
-
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1‐19. - PubMed
-
- Ettinger DS, Wood DE, Aisner DL et al., National Comprehensive Cancer Network, NCCN . clinical practice guidelines in oncology (NCCN Guidelines) non‐small cell lung cancer. Version 2.2021.
-
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389:299‐311. - PubMed
-
- Griesinger F, Eberhardt WEE, Nusch A, et al. Testing for and frequency of molecular alterations in patients with advanced NSCLC in Germany. Results from the prospective German registry CRISP (AIO‐TRK‐0315). Ann Oncol. 2018;29:viii515.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

