Generation of False-Positive SARS-CoV-2 Antigen Results with Testing Conditions outside Manufacturer Recommendations: A Scientific Approach to Pandemic Misinformation
- PMID: 34668722
- PMCID: PMC8528119
- DOI: 10.1128/Spectrum.00683-21
Generation of False-Positive SARS-CoV-2 Antigen Results with Testing Conditions outside Manufacturer Recommendations: A Scientific Approach to Pandemic Misinformation
Abstract
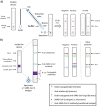
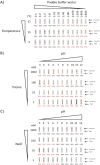
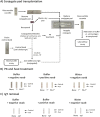
Antigen-based rapid diagnostics tests (Ag-RDTs) are useful tools for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection. However, misleading demonstrations of the Abbott Panbio coronavirus disease 2019 (COVID-19) Ag-RDT on social media claimed that SARS-CoV-2 antigen could be detected in municipal water and food products. To offer a scientific rebuttal to pandemic misinformation and disinformation, this study explored the impact of using the Panbio SARS-CoV-2 assay with conditions falling outside manufacturer recommendations. Using Panbio, various water and food products, laboratory buffers, and SARS-CoV-2-negative clinical specimens were tested with and without manufacturer buffer. Additional experiments were conducted to assess the role of each Panbio buffer component (tricine, NaCl, pH, and Tween 20) as well as the impact of temperature (4°C, 20°C, and 45°C) and humidity (90%) on assay performance. Direct sample testing (without the kit buffer) resulted in false-positive signals resembling those obtained with SARS-CoV-2 positive controls tested under proper conditions. The likely explanation of these artifacts is nonspecific interactions between the SARS-CoV-2-specific conjugated and capture antibodies, as proteinase K treatment abrogated this phenomenon, and thermal shift assays showed pH-induced conformational changes under conditions promoting artifact formation. Omitting, altering, and reverse engineering the kit buffer all supported the importance of maintaining buffering capacity, ionic strength, and pH for accurate kit function. Interestingly, the Panbio assay could tolerate some extremes of temperature and humidity outside manufacturer claims. Our data support strict adherence to manufacturer instructions to avoid false-positive SARS-CoV-2 Ag-RDT reactions, otherwise resulting in anxiety, overuse of public health resources, and dissemination of misinformation. IMPORTANCE With the Panbio severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen test being deployed in over 120 countries worldwide, understanding conditions required for its ideal performance is critical. Recently on social media, this kit was shown to generate false positives when manufacturer recommendations were not followed. While erroneous results from improper use of a test may not be surprising to some health care professionals, understanding why false positives occur can help reduce the propagation of misinformation and provide a scientific rebuttal for these aberrant findings. This study demonstrated that the kit buffer's pH, ionic strength, and buffering capacity were critical components to ensure proper kit function and avoid generation of false-positive results. Typically, false positives arise from cross-reacting or interfering substances; however, this study demonstrated a mechanism where false positives were generated under conditions favoring nonspecific interactions between the two antibodies designed for SARS-CoV-2 antigen detection. Following the manufacturer instructions is critical for accurate test results.
Keywords: COVID-19; Panbio; SARS-CoV-2; antigen; clinical methods; diagnostics; epidemiology; false positive; virology.
Figures

References
-
- Safiabadi Tali SH, LeBlanc JJ, Sadiq Z, Oyewunmi OD, Camargo C, Nikpour B, Armanfard N, Sagan SM, Jahanshahi-Anbuhi S. 2021. Tools and techniques for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 detection. Clin Microbiol Rev 34:e00228-20. doi: 10.1128/CMR.00228-20. - DOI - PMC - PubMed
-
- Public Health Agency of Canada (PHAC). 2020. Interim guidance on the use of rapid antigen detection tests for the identification of SARS-CoV-2 infection. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coro.... Accessed 9 September 2021.
-
- World Health Organization (WHO). 2020. Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays. Interim guidance. https://www.who.int/publications/i/item/antigen-detection-in-the-diagnos.... Accessed 9 September 2021.
-
- US Food and Drug Administration. 2020. In vitro diagnostics EUAs. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em.... Accessed 9 September 2021.
-
- Health Canada. 2020. Authorized medical devices for uses related to COVID-19: list of authorized testing devices. https://www.canada.ca/en/health-canada/services/drugs-health-products/co.... Accessed 9 September 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

