Multisite Clinical Validation of Isothermal Amplification-Based SARS-CoV-2 Detection Assays Using Different Sampling Strategies
- PMID: 34668736
- PMCID: PMC8528118
- DOI: 10.1128/Spectrum.00846-21
Multisite Clinical Validation of Isothermal Amplification-Based SARS-CoV-2 Detection Assays Using Different Sampling Strategies
Abstract
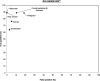
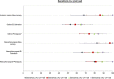
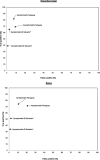
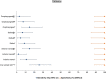
Isothermal amplification-based tests have been introduced as rapid, low-cost, and simple alternatives to real-time reverse transcriptase PCR (RT-PCR) tests for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection. The clinical performance of two isothermal amplification-based tests (Atila Biosystems iAMP coronavirus disease of 2019 [COVID-19] detection test and OptiGene COVID-19 direct plus RT-loop-mediated isothermal amplification [LAMP] test) was compared with that of clinical RT-PCR assays using different sampling strategies. A total of 1,378 participants were tested across 4 study sites. Compared with standard of care RT-PCR testing, the overall sensitivity and specificity of the Atila iAMP test for detection of SARS-CoV-2 were 76.2% and 94.9%, respectively, and increased to 88.8% and 89.5%, respectively, after exclusion of an outlier study site. Sensitivity varied based on the anatomic site from which the sample was collected. Sensitivity for nasopharyngeal sampling was 65.4% (range across study sites, 52.8% to 79.8%), for midturbinate was 88.2%, for saliva was 55.1% (range across study sites, 42.9% to 77.8%), and for anterior nares was 66.7% (range across study sites, 63.6% to 76.5%). The specificity for these anatomic collection sites ranged from 96.7% to 100%. Sensitivity improved in symptomatic patients (overall, 82.7%) and those with a higher viral load (overall, 92.4% for cycle threshold [CT] of ≤25). Sensitivity and specificity of the OptiGene direct plus RT-LAMP test, which was conducted at a single study site, were 25.5% and 100%, respectively. The Atila iAMP COVID test with midturbinate sampling is a rapid, low-cost assay for detecting SARS-CoV-2, especially in symptomatic patients and those with a high viral load, and could be used to reduce the risk of SARS-CoV-2 transmission in clinical settings. Variation of performance between study sites highlights the need for site-specific clinical validation of these assays before clinical adoption. IMPORTANCE Numerous SARS-CoV-2 detection assays have been developed and introduced into the market under emergency use authorizations (EUAs). EUAs are granted primarily based on small studies of analytic sensitivity and specificity with limited clinical validations. A thorough clinical performance evaluation of SARS-CoV-2 assays is important to understand the strengths, limitations, and specific applications of these assays. In this first large-scale multicentric study, we evaluated the clinical performance and operational characteristics of two isothermal amplification-based SARS-CoV-2 tests, namely, (i) iAMP COVID-19 detection test (Atila BioSystems, USA) and (ii) COVID-19 direct plus RT-LAMP test (OptiGene Ltd., UK), compared with those of clinical RT-PCR tests using different sampling strategies (i.e., nasopharyngeal, self-sampled anterior nares, self-sampled midturbinate, and saliva). An important specific use for these isothermal amplification-based, rapid, low-cost, and easy-to-perform SARS-CoV-2 assays is to allow for a safer return to preventive clinical encounters, such as cancer screening, particularly in low- and middle-income countries that have low SARS-CoV-2 vaccination rates.
Keywords: COVID-19; SARS-CoV-2; cancer screening; clinical validation; isothermal amplification.
Conflict of interest statement
We have nothing to declare. None of the companies had any role in design, analysis, interpretation, and finalization of the manuscript.
Figures

Update of
-
Multi-site clinical validation of Isothermal Amplification based SARS-COV-2 detection assays using different sampling strategies.medRxiv [Preprint]. 2021 Jul 6:2021.07.01.21259879. doi: 10.1101/2021.07.01.21259879. medRxiv. 2021. Update in: Microbiol Spectr. 2021 Oct 31;9(2):e0084621. doi: 10.1128/Spectrum.00846-21. PMID: 34268516 Free PMC article. Updated. Preprint.
References
-
- WHO. 2021. WHO coronavirus (COVID-19) dashboard. WHO, Geneva, Switzerland. https://covid19.who.int/.
-
- UNICEF. 2021. COVID-19 vaccine market dashboard. UNICEF, Copenhagen, Denmark. https://www.unicef.org/supply/covid-19-vaccine-market-dashboard.
-
- FDA. 2021. In vitro diagnostics EUAs—molecular diagnostic tests for SARS-CoV-2. FDA, Silver Spring, MD. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em....
-
- Mitchell SL, St. George K, Rhoads DD, Butler-Wu SM, Dharmarha V, McNult P, Miller MB, American Society for Microbiology Clinical and Public Health Microbiology Committee. 2020. Understanding, verifying, and implementing emergency use authorization molecular diagnostics for the detection of SARS-CoV-2 RNA. J Clin Microbiol 58:e00796-20. doi:10.1128/JCM.00796-20. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

