The K18-Human ACE2 Transgenic Mouse Model Recapitulates Non-severe and Severe COVID-19 in Response to an Infectious Dose of the SARS-CoV-2 Virus
- PMID: 34668775
- PMCID: PMC8754221
- DOI: 10.1128/JVI.00964-21
The K18-Human ACE2 Transgenic Mouse Model Recapitulates Non-severe and Severe COVID-19 in Response to an Infectious Dose of the SARS-CoV-2 Virus
Abstract
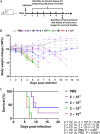
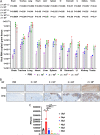
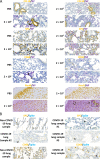
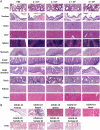
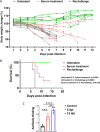
A comprehensive analysis and characterization of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection model that mimics non-severe and severe coronavirus disease 2019 (COVID-19) in humans is warranted for understating the virus and developing preventive and therapeutic agents. Here, we characterized the K18-hACE2 mouse model expressing human (h)ACE2 in mice, controlled by the human keratin 18 (K18) promoter, in the epithelia, including airway epithelial cells where SARS-CoV-2 infections typically start. We found that intranasal inoculation with higher viral doses (2 × 103 and 2 × 104 PFU) of SARS-CoV-2 caused lethality of all mice and severe damage of various organs, including lung, liver, and kidney, while lower doses (2 × 101 and 2 × 102 PFU) led to less severe tissue damage and some mice recovered from the infection. In this hACE2 mouse model, SARS-CoV-2 infection damaged multiple tissues, with a dose-dependent effect in most tissues. Similar damage was observed in postmortem samples from COVID-19 patients. Finally, the mice that recovered from infection with a low dose of virus survived rechallenge with a high dose of virus. Compared to other existing models, the K18-hACE2 model seems to be the most sensitive COVID-19 model reported to date. Our work expands the information available about this model to include analysis of multiple infectious doses and various tissues with comparison to human postmortem samples from COVID-19 patients. In conclusion, the K18-hACE2 mouse model recapitulates both severe and non-severe COVID-19 in humans being dose-dependent and can provide insight into disease progression and the efficacy of therapeutics for preventing or treating COVID-19. IMPORTANCE The pandemic of coronavirus disease 2019 (COVID-19) has reached nearly 240 million cases, caused nearly 5 million deaths worldwide as of October 2021, and has raised an urgent need for the development of novel drugs and therapeutics to prevent the spread and pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). To achieve this goal, an animal model that recapitulates the features of human COVID-19 disease progress and pathogenesis is greatly needed. In this study, we have comprehensively characterized a mouse model of SARS-CoV-2 infection using K18-hACE2 transgenic mice. We infected the mice with low and high doses of SARS-CoV-2 to study the pathogenesis and survival in response to different infection patterns. Moreover, we compared the pathogenesis of the K18-hACE2 transgenic mice with that of the COVID-19 patients to show that this model could be a useful tool for the development of antiviral drugs and therapeutics.
Keywords: COVID-19; K18-hACE2; SARS-CoV-2; infectious disease; lung infection; mouse model.
Figures
Similar articles
-
A human-ACE2 knock-in mouse model for SARS-CoV-2 infection recapitulates respiratory disorders but avoids neurological disease associated with the transgenic K18-hACE2 model.mBio. 2025 May 14;16(5):e0072025. doi: 10.1128/mbio.00720-25. Epub 2025 Apr 24. mBio. 2025. PMID: 40272151 Free PMC article.
-
SARS-CoV-2 Causes Lung Infection without Severe Disease in Human ACE2 Knock-In Mice.J Virol. 2022 Jan 12;96(1):e0151121. doi: 10.1128/JVI.01511-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34668780 Free PMC article.
-
Infectious Clones Produce SARS-CoV-2 That Causes Severe Pulmonary Disease in Infected K18-Human ACE2 Mice.mBio. 2021 Apr 20;12(2):e00819-21. doi: 10.1128/mBio.00819-21. mBio. 2021. PMID: 33879586 Free PMC article.
-
K18- and CAG-hACE2 Transgenic Mouse Models and SARS-CoV-2: Implications for Neurodegeneration Research.Molecules. 2022 Jun 28;27(13):4142. doi: 10.3390/molecules27134142. Molecules. 2022. PMID: 35807384 Free PMC article. Review.
-
Recovery scenario and immunity in COVID-19 disease: A new strategy to predict the potential of reinfection.J Adv Res. 2021 Jul;31:49-60. doi: 10.1016/j.jare.2020.12.013. Epub 2021 Jan 5. J Adv Res. 2021. PMID: 33520309 Free PMC article. Review.
Cited by
-
Animal models in SARS-CoV-2 research.Nat Methods. 2022 Apr;19(4):392-394. doi: 10.1038/s41592-022-01447-w. Nat Methods. 2022. PMID: 35396468 No abstract available.
-
SARS-CoV-2 variants induce distinct disease and impact in the bone marrow and thymus of mice.iScience. 2023 Feb 17;26(2):105972. doi: 10.1016/j.isci.2023.105972. Epub 2023 Jan 13. iScience. 2023. PMID: 36687317 Free PMC article.
-
Animal models to study the neurological manifestations of the post-COVID-19 condition.Lab Anim (NY). 2023 Sep;52(9):202-210. doi: 10.1038/s41684-023-01231-z. Epub 2023 Aug 24. Lab Anim (NY). 2023. PMID: 37620562 Free PMC article. Review.
-
Proof-of-concept studies with a computationally designed Mpro inhibitor as a synergistic combination regimen alternative to Paxlovid.Proc Natl Acad Sci U S A. 2024 Apr 23;121(17):e2320713121. doi: 10.1073/pnas.2320713121. Epub 2024 Apr 15. Proc Natl Acad Sci U S A. 2024. PMID: 38621119 Free PMC article.
-
Harnessing the power of IFN for therapeutic approaches to COVID-19.J Virol. 2024 May 14;98(5):e0120423. doi: 10.1128/jvi.01204-23. Epub 2024 Apr 23. J Virol. 2024. PMID: 38651899 Free PMC article. Review.
References
-
- WHO. 2021. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19).
-
- Steenblock C, Schwarz PEH, Ludwig B, Linkermann A, Zimmet P, Kulebyakin K, Tkachuk VA, Markov AG, Lehnert H, de Angelis MH, Rietzsch H, Rodionov RN, Khunti K, Hopkins D, Birkenfeld AL, Boehm B, Holt RIG, Skyler JS, DeVries JH, Renard E, Eckel RH, Alberti K, Geloneze B, Chan JC, Mbanya JC, Onyegbutulem HC, Ramachandran A, Basit A, Hassanein M, Bewick G, Spinas GA, Beuschlein F, Landgraf R, Rubino F, Mingrone G, Bornstein SR. 2021. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol 9:786–798. 10.1016/S2213-8587(21)00244-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI129582/AI/NIAID NIH HHS/United States
- NS106170/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 NS106170/NS/NINDS NIH HHS/United States
- R35 CA210087/CA/NCI NIH HHS/United States
- CA223400/HHS | NIH | National Cancer Institute (NCI)
- DISC2COVID19-11947/California Institute for Regenerative Medicine
- CA210087/HHS | NIH | National Cancer Institute (NCI)
- P30 CA033572/CA/NCI NIH HHS/United States
- AI129582/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- CA247550/HHS | NIH | National Cancer Institute (NCI)
- P01 CA163205/CA/NCI NIH HHS/United States
- R01 CA247550/CA/NCI NIH HHS/United States
- CA163205/HHS | NIH | National Cancer Institute (NCI)
- R21 CA223400/CA/NCI NIH HHS/United States
- 1364-19/Leukemia and Lymphoma Society (LLS)
- R01 CA266457/CA/NCI NIH HHS/United States
- R01 CA265095/CA/NCI NIH HHS/United States
- R01 AI161175/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

