SARS-CoV-2 Vaccine Induced Atypical Immune Responses in Antibody Defects: Everybody Does their Best
- PMID: 34669144
- PMCID: PMC8527979
- DOI: 10.1007/s10875-021-01133-0
SARS-CoV-2 Vaccine Induced Atypical Immune Responses in Antibody Defects: Everybody Does their Best
Abstract
Background: Data on immune responses to SARS-CoV-2 in patients with Primary Antibody Deficiencies (PAD) are limited to infected patients and to heterogeneous cohorts after immunization.
Methods: Forty-one patients with Common Variable Immune Deficiencies (CVID), six patients with X-linked Agammaglobulinemia (XLA), and 28 healthy age-matched controls (HD) were analyzed for anti-Spike and anti-receptor binding domain (RBD) antibody production, generation of Spike-specific memory B-cells, and Spike-specific T-cells before vaccination and one week after the second dose of BNT162b2 vaccine.
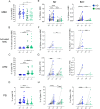
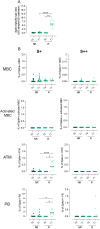
Results: The vaccine induced Spike-specific IgG and IgA antibody responses in all HD and in 20% of SARS-CoV-2 naive CVID patients. Anti-Spike IgG were detectable before vaccination in 4 out 7 CVID previously infected with SARS-CoV-2 and were boosted in six out of seven patients by the subsequent immunization raising higher levels than patients naïve to infection. While HD generated Spike-specific memory B-cells, and RBD-specific B-cells, CVID generated Spike-specific atypical B-cells, while RBD-specific B-cells were undetectable in all patients, indicating the incapability to generate this new specificity. Specific T-cell responses were evident in all HD and defective in 30% of CVID. All but one patient with XLA responded by specific T-cell only.
Conclusion: In PAD patients, early atypical immune responses after BNT162b2 immunization occurred, possibly by extra-follicular or incomplete germinal center reactions. If these responses to vaccination might result in a partial protection from infection or reinfection is now unknown. Our data suggests that SARS-CoV-2 infection more effectively primes the immune response than the immunization alone, possibly suggesting the need for a third vaccine dose for patients not previously infected.
Keywords: BNT162b2 vaccine; COVID-19; Common variable immune deficiencies; Memory cells; Primary antibody deficiencies; Receptor-binding-domain; SARS-CoV-2; Spike protein; X-linked agammaglobulinemia.
© 2021. The Author(s).
Conflict of interest statement
Authors declare that they have no conflict interest.
Figures
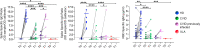

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

