Orthogonal Design of Experiments for Optimization of Lipid Nanoparticles for mRNA Engineering of CAR T Cells
- PMID: 34669421
- PMCID: PMC9335860
- DOI: 10.1021/acs.nanolett.1c02503
Orthogonal Design of Experiments for Optimization of Lipid Nanoparticles for mRNA Engineering of CAR T Cells
Abstract
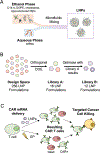
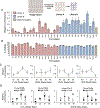
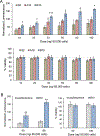
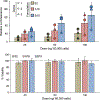
Viral engineered chimeric antigen receptor (CAR) T cell therapies are potent, targeted cancer immunotherapies, but their permanent CAR expression can lead to severe adverse effects. Nonviral messenger RNA (mRNA) CAR T cells are being explored to overcome these drawbacks, but electroporation, the most common T cell transfection method, is limited by cytotoxicity. As a potentially safer nonviral delivery strategy, here, sequential libraries of ionizable lipid nanoparticle (LNP) formulations with varied excipient compositions were screened in comparison to a standard formulation for improved mRNA delivery to T cells with low cytotoxicity, revealing B10 as the top formulation with a 3-fold increase in mRNA delivery. When compared to electroporation in primary human T cells, B10 LNPs induced comparable CAR expression with reduced cytotoxicity while demonstrating potent cancer cell killing. These results demonstrate the impact of excipient optimization on LNP performance and support B10 LNPs as a potent mRNA delivery platform for T cell engineering.
Keywords: CAR T; T cell engineering; lipid nanoparticles; mRNA delivery.
Conflict of interest statement
The authors declare the following competing financial interest(s): M.J.M., M.M.B., and C.H.J. are inventors on patents related to this work filed by the Trustees of the University of Pennsylvania (PCT/US20/56252 and PCT/ US20/56255).
Figures

References
-
- U.S. Food & Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-c... (accessed September 27, 2021).
-
- Bouchkouj N; Kasamon YL; Claro R. A. de; George B; Lin X; Lee S; Blumenthal GM; Bryan W; McKee AE; Pazdur R FDA Approval Summary: Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma. Clin. Cancer Res. 2019, 25 (6), 1702–1708. - PubMed
-
- FDA. FDA Approves First Cell-Based Gene Therapy For Adult Patients with Relapsed or Refractory MCL. U.S. Food Drug Adm. 2020, 1.
-
- U.S. Food & Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approval-brings-... (accessed September 27, 2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

