Isolation and Characterization of Lignocellulose-Degrading Geobacillus thermoleovorans from Yellowstone National Park
- PMID: 34669438
- PMCID: PMC8752141
- DOI: 10.1128/AEM.00958-21
Isolation and Characterization of Lignocellulose-Degrading Geobacillus thermoleovorans from Yellowstone National Park
Erratum in
-
Correction for Meslé et al., "Isolation and Characterization of Lignocellulose-Degrading Geobacillus thermoleovorans from Yellowstone National Park".Appl Environ Microbiol. 2022 May 10;88(9):e0059822. doi: 10.1128/aem.00598-22. Epub 2022 Apr 19. Appl Environ Microbiol. 2022. PMID: 35438518 Free PMC article. No abstract available.
Abstract
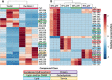
The microbial degradation of lignocellulose in natural ecosystems presents numerous biotechnological opportunities, including biofuel production from agricultural waste and feedstock biomass. To explore the degradation potential of specific thermophiles, we have identified and characterized extremophilic microorganisms isolated from hot springs environments that are capable of biodegrading lignin and cellulose substrates under thermoalkaline conditions, using a combination of culturing, genomics, and metabolomics techniques. Organisms that can use lignin and cellulose as a sole carbon source at 60 to 75°C were isolated from sediment slurry of thermoalkaline hot springs (71 to 81°C and pH 8 to 9) of Yellowstone National Park. Full-length 16S rRNA gene sequencing indicated that these isolates were closely related to Geobacillus thermoleovorans. Interestingly, most of these isolates demonstrated biofilm formation on lignin, a phenotype that is correlated with increased bioconversion. Assessment of metabolite level changes in two Geobacillus isolates from two representative springs were undertaken to characterize the metabolic responses associated with growth on glucose versus lignin carbon source as a function of pH and temperature. Overall, results from this study support that thermoalkaline springs harbor G. thermoleovorans microorganisms with lignocellulosic biomass degradation capabilities and potential downstream biotechnological applications. IMPORTANCE Since lignocellulosic biomass represents a major agro-industrial waste and renewable resource, its potential to replace nonrenewable petroleum-based products for energy production is considerable. Microbial ligninolytic and cellulolytic enzymes are of high interest in biorefineries for the valorization of lignocellulosic biomass, as they can withstand the extreme conditions (e.g., high temperature and high pH) required for processing. Of great interest is the ligninolytic potential of specific Geobacillus thermoleovorans isolates to function at a broad range of pH and temperatures, since lignin is the bottleneck in the bioprocessing of lignocellulose. In this study, results obtained from G. thermoleovorans isolates originating from YNP springs are significant because very few microorganisms from alkaline thermal environments have been discovered to have lignin- and cellulose-biodegrading capabilities, and this work opens new avenues for the biotechnological valorization of lignocellulosic biomass at an industrial scale.
Keywords: alkaline geothermal spring; biodegradation; biofuel; lignin; metabolomics.
Figures



References
-
- Anwar Z, Gulfraz M, Irshad M. 2014. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Applied Sci 7:163–173. 10.1016/j.jrras.2014.02.003. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

