Skeletal Muscle Nitrate as a Regulator of Systemic Nitric Oxide Homeostasis
- PMID: 34669624
- PMCID: PMC8677611
- DOI: 10.1249/JES.0000000000000272
Skeletal Muscle Nitrate as a Regulator of Systemic Nitric Oxide Homeostasis
Abstract
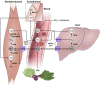
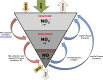
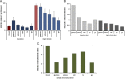
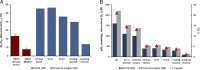
Nonenzymatic nitric oxide (NO) generation via the reduction of nitrate and nitrite ions, along with remarkably high levels of nitrate ions in skeletal muscle, have been described recently. Skeletal muscle nitrate storage may be critical for maintenance of NO homeostasis in healthy aging, and nitrate supplementation may be useful for the treatment of specific pathophysiologies and for enhancing normal functions.
Copyright © 2022 Written work prepared by employees of the Federal Government as part of their official duties is, under the U.S. Copyright Act, a "work of the United States Government" for which copyright protection under Title 17 of the United States Code is not available. As such, copyright does not extend to the contributions of employees of the Federal Government.
Figures
References
-
- Moncada S, Radomski MW, Palmer RM. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem. Pharmacol. 1988; 37(13):2495–501. - PubMed
-
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008; 7(2):156–67. - PubMed
-
- Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral Sci. 2005; 113(1):14–9. - PubMed
-
- Jansson EA Huang L Malkey R, et al. . A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008; 4(7):411–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

