Humoral and cellular responses after COVID-19 vaccination in anti-CD20-treated lymphoma patients
- PMID: 34669919
- PMCID: PMC8530768
- DOI: 10.1182/blood.2021013445
Humoral and cellular responses after COVID-19 vaccination in anti-CD20-treated lymphoma patients
Figures
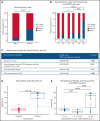
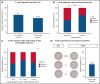
Comment in
-
COVID-specific T's may offset therapeutically endangered B's.Blood. 2022 Jan 6;139(1):12-13. doi: 10.1182/blood.2021014466. Blood. 2022. PMID: 34989773 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

