Dereplication of Bioactive Spirostane Saponins from Agave macroacantha
- PMID: 34670365
- PMCID: PMC8630797
- DOI: 10.1021/acs.jnatprod.1c00663
Dereplication of Bioactive Spirostane Saponins from Agave macroacantha
Abstract
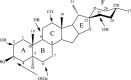
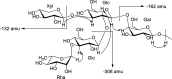
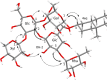
A dereplication strategy using UPLC-QTOF/MSE, the HMAI method, and NMR spectroscopy led to the identification of five main steroidal saponins (1-5), including three previously unknown compounds named macroacanthosides A-C (3-5), in a bioactive fraction of Agave macroacantha. The major saponins were isolated, and some of them together with the saponin-rich fraction were then evaluated for phytotoxicity on a standard target species, Lactuca sativa. The inhibition values exhibited by the pure compounds were confirmed to be in agreement with the phytotoxicity of the saponin-rich fraction, which suggests that the saponin fraction could be applied successfully as an agrochemical without undergoing any further costly and/or time-consuming purification processes. The NMR data of the pure compounds as well as of those corresponding to the same compounds in the fraction were comparable, which indicated that the main saponins could be identified by means of this replication workflow and that no standards are required.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Spiteller D. In Encyclopedia of Ecology; Jørgensen S. E.; Fath B. D., Eds.; Academic Press: Oxford, UK, 2008; pp 2798–2811.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

