Optogenetic stimulation of glutamatergic neurons in the cuneiform nucleus controls locomotion in a mouse model of Parkinson's disease
- PMID: 34670837
- PMCID: PMC8639376
- DOI: 10.1073/pnas.2110934118
Optogenetic stimulation of glutamatergic neurons in the cuneiform nucleus controls locomotion in a mouse model of Parkinson's disease
Abstract
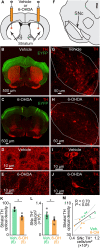
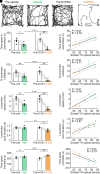
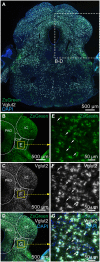
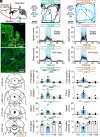
In Parkinson's disease (PD), the loss of midbrain dopaminergic cells results in severe locomotor deficits, such as gait freezing and akinesia. Growing evidence indicates that these deficits can be attributed to the decreased activity in the mesencephalic locomotor region (MLR), a brainstem region controlling locomotion. Clinicians are exploring the deep brain stimulation of the MLR as a treatment option to improve locomotor function. The results are variable, from modest to promising. However, within the MLR, clinicians have targeted the pedunculopontine nucleus exclusively, while leaving the cuneiform nucleus unexplored. To our knowledge, the effects of cuneiform nucleus stimulation have never been determined in parkinsonian conditions in any animal model. Here, we addressed this issue in a mouse model of PD, based on the bilateral striatal injection of 6-hydroxydopamine, which damaged the nigrostriatal pathway and decreased locomotor activity. We show that selective optogenetic stimulation of glutamatergic neurons in the cuneiform nucleus in mice expressing channelrhodopsin in a Cre-dependent manner in Vglut2-positive neurons (Vglut2-ChR2-EYFP mice) increased the number of locomotor initiations, increased the time spent in locomotion, and controlled locomotor speed. Using deep learning-based movement analysis, we found that the limb kinematics of optogenetic-evoked locomotion in pathological conditions were largely similar to those recorded in intact animals. Our work identifies the glutamatergic neurons of the cuneiform nucleus as a potentially clinically relevant target to improve locomotor activity in parkinsonian conditions. Our study should open avenues to develop the targeted stimulation of these neurons using deep brain stimulation, pharmacotherapy, or optogenetics.
Keywords: Parkinson’s disease; Vglut2; cuneiform nucleus; locomotion; optogenetics.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Bloem B. R., Hausdorff J. M., Visser J. E., Giladi N., Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. 19, 871–884 (2004). - PubMed
-
- Zweig R. M., et al. ., Loss of pedunculopontine neurons in progressive supranuclear palsy. Ann. Neurol. 22, 18–25 (1987). - PubMed
-
- Rinne J. O., Ma S. Y., Lee M. S., Collan Y., Röyttä M., Loss of cholinergic neurons in the pedunculopontine nucleus in Parkinson’s disease is related to disability of the patients. Parkinsonism Relat. Disord. 14, 553–557 (2008). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

