Discovery and characterization of high-affinity, potent SARS-CoV-2 neutralizing antibodies via single B cell screening
- PMID: 34671080
- PMCID: PMC8528929
- DOI: 10.1038/s41598-021-99401-x
Discovery and characterization of high-affinity, potent SARS-CoV-2 neutralizing antibodies via single B cell screening
Abstract
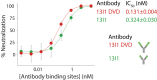
Monoclonal antibodies that target SARS-CoV-2 with high affinity are valuable for a wide range of biomedical applications involving novel coronavirus disease (COVID-19) diagnosis, treatment, and prophylactic intervention. Strategies for the rapid and reliable isolation of these antibodies, especially potent neutralizing antibodies, are critical toward improved COVID-19 response and informed future response to emergent infectious diseases. In this study, single B cell screening was used to interrogate antibody repertoires of immunized mice and isolate antigen-specific IgG1+ memory B cells. Using these methods, high-affinity, potent neutralizing antibodies were identified that target the receptor-binding domain of SARS-CoV-2. Further engineering of the identified molecules to increase valency resulted in enhanced neutralizing activity. Mechanistic investigation revealed that these antibodies compete with ACE2 for binding to the receptor-binding domain of SARS-CoV-2. These antibodies may warrant further development for urgent COVID-19 applications. Overall, these results highlight the potential of single B cell screening for the rapid and reliable identification of high-affinity, potent neutralizing antibodies for infectious disease applications.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
- R35 GM136300/GM/NIGMS NIH HHS/United States
- 1159943/National Science Foundation (NSF)
- F32 GM137513/GM/NIGMS NIH HHS/United States
- 1605266/National Science Foundation (NSF)
- R01 AI051588/AI/NIAID NIH HHS/United States
- R01 AI 51588 01/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- 1813963/National Science Foundation (NSF)
- RF1 AG059723/AG/NIA NIH HHS/United States
- RF1AG059723/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- F32 GM137513/GM/NIGMS NIH HHS/United States
- R35GM136300/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

