Tyrosinase-mediated synthesis of larvicidal active 1,5-diphenyl pent-4-en-1-one derivatives against Culex quinquefasciatus and investigation of their ichthyotoxicity
- PMID: 34671085
- PMCID: PMC8528871
- DOI: 10.1038/s41598-021-98281-5
Tyrosinase-mediated synthesis of larvicidal active 1,5-diphenyl pent-4-en-1-one derivatives against Culex quinquefasciatus and investigation of their ichthyotoxicity
Abstract
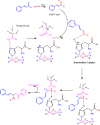
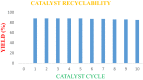
1,5-diphenylpent-4-en-1-one derivatives were synthesised using the grindstone method with Cu(II)-tyrosinase used as a catalyst. This method showed a high yield under mild reaction conditions. The synthesised compounds were identified by FTIR, 1H NMR, 13C NMR, mass spectrometry, and elemental analysis. In this study, a total of 17 compounds (1a-1q) were synthesised, and their larvicidal and antifeedant activities were evaluated. Compound 1i (1-(5-oxo-1,5-diphenylpent-1-en-3-yl)-3-(3-phenylallylidene)thiourea) was notably more active (LD50: 28.5 µM) against Culex quinquefasciatus than permethrin(54.6 µM) and temephos(37.9 µM), whereas compound 1i at 100 µM caused 0% mortality in Oreochromis mossambicus within 24 h in an antifeedant screening, with ichthyotoxicity determined as the death ratio (%) at 24 h. Compounds 1a, 1e, 1f, 1j, and 1k were found to be highly toxic, whereas 1i was not toxic in antifeedant screening. Compound 1i was found to possess a high larvicidal activity against C. quinquefasciatus and was non-toxic to non-target aquatic species. Molecular docking studies also supported the finding that 1i is a potent larvicide with higher binding energy than the control (- 10.0 vs. - 7.6 kcal/mol) in the 3OGN protein. Lead molecules are important for their larvicidal properties and application as insecticides.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
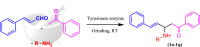





References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

