Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s
- PMID: 34671110
- PMCID: PMC9160000
- DOI: 10.1038/s41401-021-00781-7
Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s
Abstract
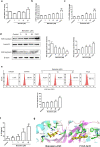
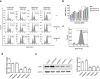
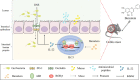
Ulcerative colitis (UC) is a chronic inflammatory disease of the gastrointestinal tract, which is closely related to gut barrier dysfunction. Emerging evidence shows that interleukin-22 (IL-22) derived from group 3 innate lymphoid cells (ILC3s) confers benefits on intestinal barrier, and IL-22 expression is controlled by aryl hydrocarbon receptor (AhR). Previous studies show that baicalein protects the colon from inflammatory damage. In this study we elucidated the molecular mechanisms underlying the protective effect of baicalein on intestinal barrier function in colitis mice. Mice were administered baicalein (10, 20, 40 mg·kg-1·d-1, i.g.) for 10 days; the mice freely drank 3% dextran sulfate sodium (DSS) on D1-D7 to induce colitis. We showed that baicalein administration simultaneously ameliorated gut inflammation, decreased intestinal permeability, restored tight junctions of colons possibly via promoting AhR/IL-22 pathway. Co-administration of AhR antagonist CH223191 (10 mg/kg, i.p.) partially blocked the therapeutic effects of baicalein in colitis mice, whereas AhR agonist FICZ (1 μg, i.p.) ameliorated symptoms and gut barrier function in colitis mice. In a murine lymphocyte line MNK-3, baicalein (5-20 μM) dose-dependently increased the expression of AhR downstream target protein CYP1A1, and enhanced IL-22 production through facilitating AhR nuclear translocation, these effects were greatly diminished in shAhR-MNK3 cells, suggesting that baicalein induced IL-22 production in AhR-dependent manner. To further clarify that, we constructed an in vitro system consisting of MNK-3 and Caco-2 cells, in which MNK-3 cell supernatant treated with baicalein could decrease FITC-dextran permeability and promoted the expression of tight junction proteins ZO-1 and occluding in Caco-2 cells. In conclusion, this study demonstrates that baicalein ameliorates colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s, thus providing a potential therapy for UC.
Keywords: CH223191; FICZ; aryl hydrocarbon receptor; baicalein; epithelial barrier; group 3 innate lymphoid cells; interleukin-22; ulcerative colitis.
© 2021. The Author(s), under exclusive licence to CPS and SIMM.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

