Preclinical study of 212Pb alpha-radioimmunotherapy targeting CD20 in non-Hodgkin lymphoma
- PMID: 34671126
- PMCID: PMC8651676
- DOI: 10.1038/s41416-021-01585-6
Preclinical study of 212Pb alpha-radioimmunotherapy targeting CD20 in non-Hodgkin lymphoma
Abstract
Background: Despite therapeutic advances, Non-Hodgkin lymphoma (NHL) relapses can occur. The development of radioimmunotherapy (RIT) with α-emitters is an attractive alternative. In this study, we investigated the potential of α-RIT in conjunction with 212Pb-rituximab for the treatment of NHL.
Methods: EL4-hCD20-Luc cells (mouse lymphoma cell line) were used for in vitro and in vivo studies. Biodistribution and efficacy studies were performed on C57BL/6 mice injected intravenously with 25 × 103 cells.
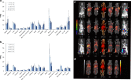
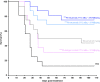
Results: 212Pb-rituximab (0.925-7.4 kBq/mL) inhibit proliferation of EL4-hCD20-Luc cells in vitro. Biodistribution of 203/212Pb-rituximab in mice showed a significant tumour uptake and suggested that the liver, spleen, and kidneys were the organs at risk. For efficacy studies, mice were treated at either 11 days (early stage) or 20-30 days after injection of tumour cells (late stage). Treatment with 277.5 kBq 212Pb-rituximab significantly prolonged survival. Even at an advanced tumour stage, significant tumour regression occurred, with an increase in the median survival time to 28 days, compared with 9 days in the controls.
Conclusions: These results show the efficacy of 212Pb-rituximab in a murine syngeneic lymphoma model, in terms of significant tumour regression and increased survival, thereby highlighting the potency of α-RIT for the treatment of NHL.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Amal Saidi and Julien Torgue are Orano Med employees. No other potential conflicts of interest relevant to this article exist.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

