Low glycaemic diets alter lipid metabolism to influence tumour growth
- PMID: 34671163
- PMCID: PMC8628459
- DOI: 10.1038/s41586-021-04049-2
Low glycaemic diets alter lipid metabolism to influence tumour growth
Abstract
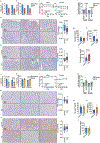
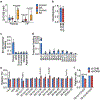
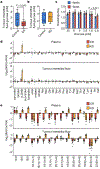
Dietary interventions can change metabolite levels in the tumour microenvironment, which might then affect cancer cell metabolism to alter tumour growth1-5. Although caloric restriction (CR) and a ketogenic diet (KD) are often thought to limit tumour progression by lowering blood glucose and insulin levels6-8, we found that only CR inhibits the growth of select tumour allografts in mice, suggesting that other mechanisms contribute to tumour growth inhibition. A change in nutrient availability observed with CR, but not with KD, is lower lipid levels in the plasma and tumours. Upregulation of stearoyl-CoA desaturase (SCD), which synthesises monounsaturated fatty acids, is required for cancer cells to proliferate in a lipid-depleted environment, and CR also impairs tumour SCD activity to cause an imbalance between unsaturated and saturated fatty acids to slow tumour growth. Enforcing cancer cell SCD expression or raising circulating lipid levels through a higher-fat CR diet confers resistance to the effects of CR. By contrast, although KD also impairs tumour SCD activity, KD-driven increases in lipid availability maintain the unsaturated to saturated fatty acid ratios in tumours, and changing the KD fat composition to increase tumour saturated fatty acid levels cooperates with decreased tumour SCD activity to slow tumour growth. These data suggest that diet-induced mismatches between tumour fatty acid desaturation activity and the availability of specific fatty acid species determine whether low glycaemic diets impair tumour growth.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Figures


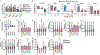





Comment in
-
Diet comparison suggests a lipid imbalance can slow tumour growth.Nature. 2021 Nov;599(7884):206-207. doi: 10.1038/d41586-021-02775-1. Nature. 2021. PMID: 34671149 No abstract available.
-
Dinner is served, sir: Fighting cancer with the right diet.Cell. 2021 Dec 22;184(26):6226-6228. doi: 10.1016/j.cell.2021.11.036. Cell. 2021. PMID: 34942098
References
Publication types
MeSH terms
Substances
Grants and funding
- K99 CA234221/CA/NCI NIH HHS/United States
- T32 GM007287/GM/NIGMS NIH HHS/United States
- U01 CA210171/CA/NCI NIH HHS/United States
- P01 CA087969/CA/NCI NIH HHS/United States
- R01 CA168653/CA/NCI NIH HHS/United States
- U01 CA176726/CA/NCI NIH HHS/United States
- R03 CA223619/CA/NCI NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- UM1 CA186107/CA/NCI NIH HHS/United States
- P50 CA127003/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- U01 CA167552/CA/NCI NIH HHS/United States
- R01 CA201276/CA/NCI NIH HHS/United States
- R35 CA242379/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

