Diagnostic and Prognostic Potential of 18F-FET PET in the Differential Diagnosis of Glioma Recurrence and Treatment-Induced Changes After Chemoradiation Therapy
- PMID: 34671551
- PMCID: PMC8521061
- DOI: 10.3389/fonc.2021.721821
Diagnostic and Prognostic Potential of 18F-FET PET in the Differential Diagnosis of Glioma Recurrence and Treatment-Induced Changes After Chemoradiation Therapy
Abstract
Background: MRI-based differential diagnosis of glioma recurrence (GR) and treatment-induced changes (TICs) remain elusive in up to 30% of treated glioma patients. We aimed to determine 18F-FET PET diagnostic performance in this clinical scenario, its outcome dependency on established prognostic factors, optimal 18F-FET semi-quantitative thresholds, and whether 18F-FET parameters may instantly predict progression-free survival (PFS) and overall survival (OS).
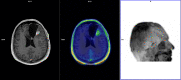
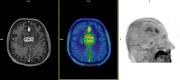
Methods: We retrospectively analyzed 45 glioma patients treated with chemoradiation therapy (32 males; mean age: 51 years, glioma grade: n=26 WHO4; n=15 WHO3; n=4 WHO2) who underwent 18F-FET PET to resolve differential diagnosis of GR and TICs raised by MRI performed in the preceding 2 weeks and depicting any of the following changes in their radiation field: volumetric increase of contrast-enhancing lesions; new contrast-enhancing lesion; significant increase in T2/FLAIR non-enhancing lesion without reducing corticosteroids. 18F-FET PET outcome relied on evaluation of maximum tumor-to-brain ratio (TBRmax), time-to-peak (TTP), and time-activity curve pattern (TAC). Metabolic tumor volume (MTV) and total tumor metabolism (TTM) were calculated for prognostic purposes. Standard of reference was repeat MRI performed 4-6 weeks after the previous MRI. Non-parametric statistics tested 18F-FET-based parameters for dependency on established prognostic markers. ROC curve analysis determined optimal cutoff values for 18F-FET semi-quantitative parameters. 18F-FET parameters and prognostic factors were evaluated for PFS and OS by Kaplan-Meier, univariate, and multivariate analyses.
Results: 18F-FET PET sensitivity, specificity, positive predictive value, negative predictive value were 86.2, 81.3, 89.3, 76.5%, respectively; higher diagnostic accuracy was yielded in IDH-wild-type glioma patients compared to IDH-mutant glioma patients (sensitivity: 81.8 versus 88.9%; specificity: 80.8 versus 81.8%). KPS was the only prognostic factor differing according to 18F-FET PET outcome (negative versus positive). Optimal 18F-FET cutoff values for GR were TBRmax ≥ 2.1, SUVmax ≥ 3.5, and TTP ≤ 29 min. PFS differed based on 18F-FET outcome and related metrics and according to KPS; a different OS was observed according to KPS only. On multivariate analysis, 18F-FET PET outcome was the only significant PFS factor; KPS and age the only significant OS factors.
Conclusion: 18F-FET PET demonstrated good diagnostic performance. 18F-FET PET outcome and metrics were significantly predictive only for PFS.
Keywords: 18F-FET PET; metabolic tumor volume; total tumor metabolism; treated gliomas; treatment-related changes.
Copyright © 2021 Celli, Caroli, Amadori, Arpa, Gurrieri, Ghigi, Cenni, Paganelli and Matteucci.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Prediction of survival in patients with IDH-wildtype astrocytic gliomas using dynamic O-(2-[18F]-fluoroethyl)-L-tyrosine PET.Eur J Nucl Med Mol Imaging. 2020 Jun;47(6):1486-1495. doi: 10.1007/s00259-020-04695-0. Epub 2020 Feb 7. Eur J Nucl Med Mol Imaging. 2020. PMID: 32034446 Free PMC article.
-
Prognostic value of pre-irradiation FET PET in patients with not completely resectable IDH-wildtype glioma and minimal or absent contrast enhancement.Sci Rep. 2021 Oct 21;11(1):20828. doi: 10.1038/s41598-021-00193-x. Sci Rep. 2021. PMID: 34675225 Free PMC article.
-
Non-invasive prediction of IDH-wildtype genotype in gliomas using dynamic 18F-FET PET.Eur J Nucl Med Mol Imaging. 2019 Nov;46(12):2581-2589. doi: 10.1007/s00259-019-04477-3. Epub 2019 Aug 13. Eur J Nucl Med Mol Imaging. 2019. PMID: 31410540
-
Diagnostic Accuracy of PET for Differentiating True Glioma Progression From Post Treatment-Related Changes: A Systematic Review and Meta-Analysis.Front Neurol. 2021 May 20;12:671867. doi: 10.3389/fneur.2021.671867. eCollection 2021. Front Neurol. 2021. PMID: 34093419 Free PMC article.
-
Contribution of [18F]FET PET in the Management of Gliomas, from Diagnosis to Follow-Up: A Review.Pharmaceuticals (Basel). 2024 Sep 18;17(9):1228. doi: 10.3390/ph17091228. Pharmaceuticals (Basel). 2024. PMID: 39338390 Free PMC article. Review.
Cited by
-
Pre-operative dual-time-point [18F]FET PET differentiates CDKN2A/B loss and PIK3CA mutation status in adult-type diffuse glioma: a single-center prospective study.Eur J Nucl Med Mol Imaging. 2025 Jan;52(2):669-682. doi: 10.1007/s00259-024-06935-z. Epub 2024 Oct 4. Eur J Nucl Med Mol Imaging. 2025. PMID: 39365462
-
Imaging-Based Patterns of Failure following Re-Irradiation for Recurrent/Progressive High-Grade Glioma.J Pers Med. 2023 Apr 19;13(4):685. doi: 10.3390/jpm13040685. J Pers Med. 2023. PMID: 37109071 Free PMC article.
-
Individualized discrimination of tumor progression from treatment-related changes in different types of adult-type diffuse gliomas using [11C]methionine PET.J Neurooncol. 2023 Dec;165(3):547-559. doi: 10.1007/s11060-023-04529-7. Epub 2023 Dec 14. J Neurooncol. 2023. PMID: 38095773
-
Clinical applications and prospects of PET imaging in patients with IDH-mutant gliomas.J Neurooncol. 2023 May;162(3):481-488. doi: 10.1007/s11060-022-04218-x. Epub 2022 Dec 29. J Neurooncol. 2023. PMID: 36577872 Free PMC article. Review.
-
Interobserver ground-truth variability limits performance of automated glioblastoma segmentation on [18F]FET PET.EJNMMI Phys. 2025 Jun 6;12(1):54. doi: 10.1186/s40658-025-00767-y. EJNMMI Phys. 2025. PMID: 40478497 Free PMC article.
References
LinkOut - more resources
Full Text Sources

