Toll-Like Receptors as a Therapeutic Target in the Era of Immunotherapies
- PMID: 34671606
- PMCID: PMC8522911
- DOI: 10.3389/fcell.2021.756315
Toll-Like Receptors as a Therapeutic Target in the Era of Immunotherapies
Abstract
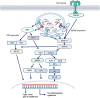
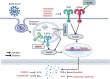
Toll-like receptors (TLRs) are the pattern recognition receptors, which are activated by foreign and host molecules in order to initiate the immune response. They play a crucial role in the regulation of innate immunity, and several studies have shown their importance in bacterial, viral, and fungal infections, autoimmune diseases, and cancers. The consensus view from an immunological perspective is that TLR agonists can serve either as a possible therapeutic agent or as a vaccine adjuvant toward cancers or infectious diseases and that TLR inhibitors may be a promising approach to the treatment of autoimmune diseases, some cancers, bacterial, and viral infections. These notions are based on the fact that TLR agonists stimulate the secretion of proinflammatory cytokines and in general, the development of proinflammatory responses. Some of the TLR-based inhibitory agents have shown to be efficacious in preclinical models and have now entered clinical trials. Therefore, TLRs seem to hold the potential to serve as a perfect target in the era of immunotherapies. We offer a perspective on TLR-based therapeutics that sheds light on their usefulness and on combination therapies. We also highlight various therapeutics that are in the discovery phase or in clinical trials.
Keywords: SARS-CoV-2; TLR-based immunotherapies; Toll-like receptor; autoimmune disorder; cancer; infection.
Copyright © 2021 Farooq, Batool, Kim and Choi.
Conflict of interest statement
MB and SC was employed by company S&K Therapeutics, South Korea. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

