Ferroptosis-Related Genes in Bronchoalveolar Lavage Fluid Serves as Prognostic Biomarkers for Idiopathic Pulmonary Fibrosis
- PMID: 34671612
- PMCID: PMC8520927
- DOI: 10.3389/fmed.2021.693959
Ferroptosis-Related Genes in Bronchoalveolar Lavage Fluid Serves as Prognostic Biomarkers for Idiopathic Pulmonary Fibrosis
Abstract
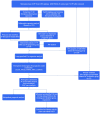
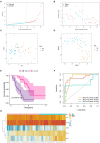
Background: Idiopathic pulmonary fibrosis (IPF) is a chronic progressive disease with unknown etiology and unfavorable prognosis. Ferroptosis is a form of regulated cell death with an iron-dependent way that is involved in the development of various diseases. Whereas the prognostic value of ferroptosis-related genes (FRGs) in IPF remains uncertain and needs to be further elucidated. Methods: The FerrDb database and the previous studies were screened to explore the FRGs. The data of patients with IPF were obtained from the GSE70866 dataset. Wilcoxon's test and univariate Cox regression analysis were applied to identify the FRGs that are differentially expressed between normal and patients with IPF and associated with prognosis. Next, a multigene signature was constructed by the least absolute shrinkage and selection operator (LASSO)-penalized Cox model in the training cohort and evaluated by using calibration and receiver operating characteristic (ROC) curves. Then, 30% of the dataset samples were randomly selected for internal validation. Finally, the potential function and pathways that might be affected by the risk score-related differently expressed genes (DEGs) were further explored. Results: A total of 183 FRGs were identified by the FerrDb database and the previous studies, and 19 of them were differentially expressed in bronchoalveolar lavage fluid (BALF) between IPF and healthy controls and associated with prognosis (p < 0.05). There were five FRGs (aconitase 1 [ACO1], neuroblastoma RAS viral (v-ras) oncogene homolog [NRAS], Ectonucleotide pyrophosphatase/phosphodiesterase 2 [ENPP2], Mucin 1 [MUC1], and ZFP36 ring finger protein [ZFP36]) identified as risk signatures and stratified patients with IPF into the two risk groups. The overall survival rate in patients with high risk was significantly lower than that in patients with low risk (p < 0.001). The calibration and ROC curve analysis confirmed the predictive capacity of this signature, and the results were further verified in the validation group. Risk score-related DEGs were found enriched in ECM-receptor interaction and focal adhesion pathways. Conclusion: The five FRGs in BALF can be used for prognostic prediction in IPF, which may contribute to improving the management strategies of IPF.
Keywords: ferroptosis; idiopathic pulmonary fibrosis; model; prognostic; signature.
Copyright © 2021 Li, Wang, Zhang, Fan, Li, Zhou, Yang, Shi, Li, Zhang, Chen and Ren.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
An 8-ferroptosis-related genes signature from Bronchoalveolar Lavage Fluid for prognosis in patients with idiopathic pulmonary fibrosis.BMC Pulm Med. 2022 Jan 5;22(1):15. doi: 10.1186/s12890-021-01799-7. BMC Pulm Med. 2022. PMID: 34983465 Free PMC article.
-
Identification and validation of autophagy-related gene expression for predicting prognosis in patients with idiopathic pulmonary fibrosis.Front Immunol. 2022 Sep 20;13:997138. doi: 10.3389/fimmu.2022.997138. eCollection 2022. Front Immunol. 2022. PMID: 36211385 Free PMC article.
-
Identification of the ferroptosis-related long non-coding RNAs signature to improve the prognosis prediction and immunotherapy response in patients with NSCLC.BMC Med Genomics. 2021 Dec 3;14(1):286. doi: 10.1186/s12920-021-01133-4. BMC Med Genomics. 2021. PMID: 34861872 Free PMC article.
-
PCR array analysis reveals a novel expression profile of ferroptosis-related genes in idiopathic pulmonary fibrosis.BMC Pulm Med. 2025 Feb 28;25(1):98. doi: 10.1186/s12890-025-03555-7. BMC Pulm Med. 2025. PMID: 40022042 Free PMC article.
-
Ferroptosis-Associated Classifier and Indicator for Prognostic Prediction in Cutaneous Melanoma.J Oncol. 2021 Oct 28;2021:3658196. doi: 10.1155/2021/3658196. eCollection 2021. J Oncol. 2021. PMID: 34745259 Free PMC article. Review.
Cited by
-
Prognostic Function and Immunologic Landscape of a Predictive Model Based on Five Senescence-Related Genes in IPF Bronchoalveolar Lavage Fluid.Biomedicines. 2024 Jun 3;12(6):1246. doi: 10.3390/biomedicines12061246. Biomedicines. 2024. PMID: 38927453 Free PMC article.
-
Prognostic value of tripartite motif (TRIM) family gene signature from bronchoalveolar lavage cells in idiopathic pulmonary fibrosis.BMC Pulm Med. 2022 Dec 6;22(1):467. doi: 10.1186/s12890-022-02269-4. BMC Pulm Med. 2022. PMID: 36474231 Free PMC article.
-
Multifaceted Roles of Ferroptosis in Lung Diseases.Front Mol Biosci. 2022 Jun 24;9:919187. doi: 10.3389/fmolb.2022.919187. eCollection 2022. Front Mol Biosci. 2022. PMID: 35813823 Free PMC article. Review.
-
Identification of ATG7 as a Regulator of Proferroptosis and Oxidative Stress in Osteosarcoma.Oxid Med Cell Longev. 2022 Oct 8;2022:8441676. doi: 10.1155/2022/8441676. eCollection 2022. Oxid Med Cell Longev. 2022. Retraction in: Oxid Med Cell Longev. 2023 Jun 21;2023:9819050. doi: 10.1155/2023/9819050. PMID: 36254233 Free PMC article. Retracted.
-
Identification of three hub genes related to the prognosis of idiopathic pulmonary fibrosis using bioinformatics analysis.Int J Med Sci. 2022 Aug 15;19(9):1417-1429. doi: 10.7150/ijms.73305. eCollection 2022. Int J Med Sci. 2022. PMID: 36035368 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

