Pediatric Clinical Trials in Mainland China Over the Past Decade (From 2009 to 2020)
- PMID: 34671625
- PMCID: PMC8520938
- DOI: 10.3389/fmed.2021.745676
Pediatric Clinical Trials in Mainland China Over the Past Decade (From 2009 to 2020)
Abstract
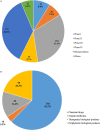
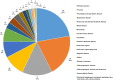
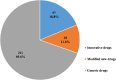
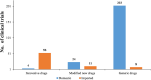
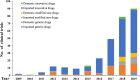
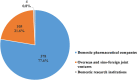
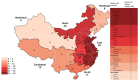
In mainland China, there remains a shortage of pediatric drugs. The Chinese government has recently launched policies and incentives to encourage pediatric drug development and clinical trials. However, data on the characteristics or development trends of these trials are limited. In this review, we extracted source data from the Chinese Clinical Trials Registry and Information Transparency Platform and systematically reviewed the pediatric clinical trials conducted in mainland China from 2009 to 2020, a comprehensive process evaluation of the pediatric drug clinical trials development in the past decade, providing data support to policy makers and industry stakeholders. We included 487 pediatric clinical trials. Over the past decade, the number of pediatric trials has increased, especially since 2016. The most common therapeutic areas were infectious diseases (n = 108, 22.2%), agents for preventive purpose (n = 99, 20.3%), and neurological and psychiatric diseases (n = 71, 14.6%). The number of clinical trials involving epilepsy (39, 10.1%), asthma (33, 8.5%), and influenza (24, 6.2%) were the highest. The distribution of leading institutions is unbalanced in mainland China, with most units in East China (34.0%) and few in Southwest China (6.9%). China has made progress in improving the research and development environment of pediatric drugs and increasing pediatric trials. However, a wide gap in pediatric drug development and clinical trials quality exists between China and the developed countries. The pharmaceutical industry in China has faced grim setbacks, including study duplication, lack of innovation, poor research design, and unbalanced resource allocation. Thus, we suggest that the Chinese government should adjust their policies to improve innovation and clinical design capacity, and optimize resource allocation between regions.
Keywords: Chinese Clinical Trials Registry and Information Transparency Platform; National Medical Products Administration; drug research and development; pediatric clinical trials; pediatric population.
Copyright © 2021 Wu, Ji, Wang, Chen, Ding, Zhang, Li, Wang, Ni, Liu, Xu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Trends in innovative pediatric drug development in China based on clinical trial registration data.Front Med (Lausanne). 2023 Jul 6;10:1187547. doi: 10.3389/fmed.2023.1187547. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37484857 Free PMC article.
-
Changes in clinical trials of cancer drugs in mainland China over the decade 2009-18: a systematic review.Lancet Oncol. 2019 Nov;20(11):e619-e626. doi: 10.1016/S1470-2045(19)30491-7. Lancet Oncol. 2019. PMID: 31674320
-
The changing landscape of anti-lymphoma drug clinical trials in mainland China in the past 15 years (2005-2020): A systematic review.Lancet Reg Health West Pac. 2021 Feb 5;8:100097. doi: 10.1016/j.lanwpc.2021.100097. eCollection 2021 Mar. Lancet Reg Health West Pac. 2021. PMID: 34327425 Free PMC article.
-
Evolution of ischemic stroke drug clinical trials in mainland China from 2005 to 2021.CNS Neurosci Ther. 2022 Aug;28(8):1229-1239. doi: 10.1111/cns.13867. Epub 2022 Jun 1. CNS Neurosci Ther. 2022. PMID: 35642775 Free PMC article.
-
Trends of Phase I Clinical Trials of New Drugs in Mainland China Over the Past 10 Years (2011-2020).Front Med (Lausanne). 2021 Dec 17;8:777698. doi: 10.3389/fmed.2021.777698. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34977078 Free PMC article.
Cited by
-
The Changing Landscape of Heart Failure Drug Clinical Trials in China, 2013-2023.Drug Des Devel Ther. 2025 Apr 3;19:2597-2608. doi: 10.2147/DDDT.S511608. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40196754 Free PMC article. Review.
-
Characteristic analysis of clinical trials for new traditional Chinese medicines in mainland China from 2013 to 2021.Front Med (Lausanne). 2022 Oct 18;9:1008683. doi: 10.3389/fmed.2022.1008683. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36330068 Free PMC article. Review.
-
Trends in innovative pediatric drug development in China based on clinical trial registration data.Front Med (Lausanne). 2023 Jul 6;10:1187547. doi: 10.3389/fmed.2023.1187547. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37484857 Free PMC article.
-
Informed Consent in Clinical Studies Involving Human Participants: Ethical Insights of Medical Researchers in Germany and Poland.Front Med (Lausanne). 2022 May 19;9:901059. doi: 10.3389/fmed.2022.901059. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35665330 Free PMC article.
References
-
- White Paper on the Investigation of Pediatric Drug Use Safety . (2016). Available online at: https://www.ruiwen.com/gongwen/diaochabaogao/99076.html (accessed April 30, 2020).
-
- Li J, Yan K, Kong YT, Ye XF, Ge MM, Zhang CF. A cross-sectional study of children clinical trials registration in the world based on ClinicalTrials.gov establishment. Chin J Evid Based Pediatr. (2016) 11:3–7. 10.3969/j.issn.1673-5501.2016.01.002 - DOI
-
- Zhang GD, Yang Y, Zhao RL. Characteristics Analysis of Pediatric Drug Clinical Trials Registered in Chinese Clinical Trial Registry. China Pharmacy. (2020) 31:2055–60. 10.6039/j.issn.1001-0408.2020.17.02 - DOI
-
- National Health Commission of the People's Republic of China . Chinese National Formulary. (2010). Available online at: http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=45875 (accessed April 30, 2020).
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

