Benefit of Bovine Viral Diarrhoea (BVD) Eradication in Cattle on Pestivirus Seroprevalence in Sheep
- PMID: 34671657
- PMCID: PMC8520948
- DOI: 10.3389/fvets.2021.681559
Benefit of Bovine Viral Diarrhoea (BVD) Eradication in Cattle on Pestivirus Seroprevalence in Sheep
Abstract
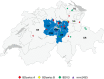
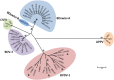
Bovine viral diarrhoea virus (BVDV) and Border disease virus (BDV) are closely related pestiviruses of cattle and sheep, respectively. Both viruses may be transmitted between either species, but control programs are restricted to BVDV in cattle. In 2008, a program to eradicate bovine viral diarrhoea (BVD) in cattle was started in Switzerland. As vaccination is prohibited, the cattle population is now widely naïve to pestivirus infections. In a recent study, we determined that nearly 10% of cattle are positive for antibodies to BDV. Here, we show that despite this regular transmission of BDV from small ruminants to cattle, we could only identify 25 cattle that were persistently infected with BDV during the last 12 years of the eradication program. In addition, by determining the BVDV and BDV seroprevalence in sheep in Central Switzerland before and after the start of the eradication, we provide evidence that BVDV is transmitted from cattle to sheep, and that the BVDV seroprevalence in sheep significantly decreased after its eradication in cattle. While BDV remains endemic in sheep, the population thus profited at least partially from BVD eradication in cattle. Importantly, on a national level, BVD eradication does not appear to be generally derailed by the presence of pestiviruses in sheep. However, with every single virus-positive cow, it is necessary to consider small ruminants as a potential source of infection, resulting in costly but essential investigations in the final stages of the eradication program.
Keywords: Switzerland; border disease virus (BDV); bovine viral diarrhoea virus (BVDV); eradication; persistent infection; pestivirus; seroprevalence; virus transmission.
Copyright © 2021 Huser, Schär, Bachofen, de Martin, Portmann, Stalder and Schweizer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Influence of border disease virus (BDV) on serological surveillance within the bovine virus diarrhea (BVD) eradication program in Switzerland.BMC Vet Res. 2017 Jan 13;13(1):21. doi: 10.1186/s12917-016-0932-0. BMC Vet Res. 2017. PMID: 28086880 Free PMC article.
-
[Pestiviruses in sheep and goats in Austria: Options for integration into the bovine viral diarrhea (BVDV) monitoring program].Schweiz Arch Tierheilkd. 2023 Dec;165(12):783-791. doi: 10.17236/sat00413. Schweiz Arch Tierheilkd. 2023. PMID: 38014544 German.
-
Eradication of Bovine Viral Diarrhoea (BVD) in Cattle in Switzerland: Lessons Taught by the Complex Biology of the Virus.Front Vet Sci. 2021 Sep 7;8:702730. doi: 10.3389/fvets.2021.702730. eCollection 2021. Front Vet Sci. 2021. PMID: 34557540 Free PMC article. Review.
-
Border disease in cattle.Vet J. 2019 Apr;246:12-20. doi: 10.1016/j.tvjl.2019.01.006. Epub 2019 Feb 1. Vet J. 2019. PMID: 30902184 Review.
-
Investigation of border disease and bovine virus diarrhoea in sheep from 76 mixed cattle and sheep farms in eastern Switzerland.Schweiz Arch Tierheilkd. 2013 May;155(5):293-8. doi: 10.1024/0036-7281/a000460. Schweiz Arch Tierheilkd. 2013. PMID: 23644292
Cited by
-
International proficiency trial for bovine viral diarrhea virus (BVDV) antibody detection: limitations of milk serology.BMC Vet Res. 2022 May 6;18(1):168. doi: 10.1186/s12917-022-03265-w. BMC Vet Res. 2022. PMID: 35524302 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

