Randomised clinical trial: A phase 2 double-blind study of namodenoson in non-alcoholic fatty liver disease and steatohepatitis
- PMID: 34671996
- PMCID: PMC9298378
- DOI: 10.1111/apt.16664
Randomised clinical trial: A phase 2 double-blind study of namodenoson in non-alcoholic fatty liver disease and steatohepatitis
Abstract
Background: Namodenoson, an A3 adenosine receptor (A3AR) agonist, improved liver function/pathology in non-alcoholic steatohepatitis (NASH) preclinical models.
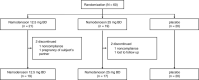
Aim: To evaluate the efficacy and safety of namodenoson for the treatment of non-alcoholic fatty liver disease (NAFLD) with or without NASH METHODS: This phase 2 study included 60 patients with NAFLD (ALT ≥60 IU/L) who were randomised (1:1:1) to oral namodenoson 12.5 mg b.d. (n = 21), 25 mg b.d. (n = 19), or placebo (n = 20) for 12 weeks (total follow-up: 16 weeks). The main efficacy endpoint involved serum ALT after 12 weeks of treatment.
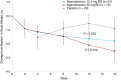
Results: Serum ALT decreased over time with namodenoson in a dose-dependent manner. The difference between change from baseline (CFB) for ALT in the namodenoson 25 mg b.d. arm vs placebo trended towards significance at 12 weeks (P = 0.066). Serum AST levels also decreased with namodenoson in a dose-dependent manner; at 12 weeks, the CFB for 25 mg b.d. vs placebo was significant (P = 0.03). At Week 12, 31.6% in the namodenoson 25 mg b.d. arm and 20.0% in the placebo arm achieved ALT normalisation (P = 0.405). At week 16, the respective rates were 36.8% and 10.0% (P = 0.038). A3AR expression levels were stable over time across study arms. Both doses of namodenoson were well tolerated with no drug-emergent severe adverse events, drug-drug interactions, hepatotoxicity, or deaths. Three adverse events were considered possibly related to study treatment: myalgia (12.5 mg b.d. arm), muscular weakness (25 mg b.d. arm), and headache (25 mg b.d. arm).
Conclusion: A3AR is a valid target; namodenoson 25 mg b.d. was safe and demonstrated efficacy signals (ClinicalTrials.gov #NCT02927314).
Keywords: clinical trial; fibrosis; liver; non-alcoholic fatty liver disease; non-alcoholic steatohepatitis.
© 2021 Can-Fite BioPharma Ltd. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures


Comment in
-
Editorial: targeting aberrant hepatic inflammation for treatment of non-alcoholic steatohepatitis-authors' reply.Aliment Pharmacol Ther. 2022 Feb;55(4):485-486. doi: 10.1111/apt.16762. Aliment Pharmacol Ther. 2022. PMID: 35092054 No abstract available.
-
Editorial: targeting aberrant hepatic inflammation for treatment of non-alcoholic steatohepatitis.Aliment Pharmacol Ther. 2022 Feb;55(4):483-484. doi: 10.1111/apt.16748. Aliment Pharmacol Ther. 2022. PMID: 35092056 No abstract available.
References
-
- Ratziu V, Bellentani S, Cortez‐Pinto H, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372‐384. - PubMed
-
- Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle‐aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124‐131. - PubMed
-
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non‐alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005‐2023. - PubMed
-
- Kabbany MN, Selvakumar PKC, Watt K, et al. Prevalence of nonalcoholic steatohepatitis‐associated cirrhosis in the United States: an analysis of national health and nutrition examination survey data. Am J Gastroenterol. 2017;112:581‐587. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
