Late-Stage Amination of Drug-Like Benzoic Acids: Access to Anilines and Drug Conjugates through Directed Iridium-Catalyzed C-H Activation
- PMID: 34672032
- PMCID: PMC9299223
- DOI: 10.1002/chem.202103510
Late-Stage Amination of Drug-Like Benzoic Acids: Access to Anilines and Drug Conjugates through Directed Iridium-Catalyzed C-H Activation
Abstract
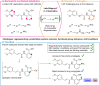
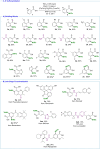
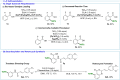
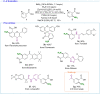
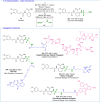
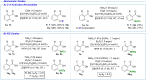
The functionalization of C-H bonds, ubiquitous in drugs and drug-like molecules, represents an important synthetic strategy with the potential to streamline the drug-discovery process. Late-stage aromatic C-N bond-forming reactions are highly desirable, but despite their significance, accessing aminated analogues through direct and selective amination of C-H bonds remains a challenging goal. The method presented herein enables the amination of a wide array of benzoic acids with high selectivity. The robustness of the system is manifested by the large number of functional groups tolerated, which allowed the amination of a diverse array of marketed drugs and drug-like molecules. Furthermore, the introduction of a synthetic handle enabled expeditious access to targeted drug-delivery conjugates, PROTACs, and probes for chemical biology. This rapid access to valuable analogues, combined with operational simplicity and applicability to high-throughput experimentation has the potential to aid and considerably accelerate drug discovery.
Keywords: C−H activation; amination; conjugation; high-throughput experimentation; iridium.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Merging Directed C-H Activations with High-Throughput Experimentation: Development of Iridium-Catalyzed C-H Aminations Applicable to Late-Stage Functionalization.JACS Au. 2022 Apr 13;2(4):906-916. doi: 10.1021/jacsau.2c00039. eCollection 2022 Apr 25. JACS Au. 2022. PMID: 35557751 Free PMC article.
-
Practical Access to meta-Substituted Anilines by Amination of Quinone Imine Ketals Derived from Anisidines: Efficient Synthesis of Anti-Psychotic Drugs.Angew Chem Int Ed Engl. 2023 May 15;62(21):e202301166. doi: 10.1002/anie.202301166. Epub 2023 Apr 14. Angew Chem Int Ed Engl. 2023. PMID: 36942400
-
Picolinate-Directed Arene meta-C-H Amination via FeCl3 Catalysis.J Am Chem Soc. 2020 Mar 18;142(11):5266-5271. doi: 10.1021/jacs.9b13753. Epub 2020 Mar 4. J Am Chem Soc. 2020. PMID: 32090542
-
The emergence of the C-H functionalization strategy in medicinal chemistry and drug discovery.Chem Commun (Camb). 2021 Oct 19;57(83):10842-10866. doi: 10.1039/d1cc04083a. Chem Commun (Camb). 2021. PMID: 34596175 Review.
-
Directing group strategies in rhodium-catalyzed C-H amination.Org Biomol Chem. 2022 Oct 5;20(38):7554-7576. doi: 10.1039/d2ob01157c. Org Biomol Chem. 2022. PMID: 36112051 Review.
Cited by
-
Late-stage modification of bioactive compounds: Improving druggability through efficient molecular editing.Acta Pharm Sin B. 2024 Mar;14(3):1030-1076. doi: 10.1016/j.apsb.2023.11.021. Epub 2023 Nov 18. Acta Pharm Sin B. 2024. PMID: 38487004 Free PMC article. Review.
-
Merging Directed C-H Activations with High-Throughput Experimentation: Development of Iridium-Catalyzed C-H Aminations Applicable to Late-Stage Functionalization.JACS Au. 2022 Apr 13;2(4):906-916. doi: 10.1021/jacsau.2c00039. eCollection 2022 Apr 25. JACS Au. 2022. PMID: 35557751 Free PMC article.
-
Synthesis of Benzoic Acids from Electrochemically Reduced CO2 Using Heterogeneous Catalysts.ChemSusChem. 2025 Feb 1;18(3):e202401084. doi: 10.1002/cssc.202401084. Epub 2024 Nov 5. ChemSusChem. 2025. PMID: 39310956 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

