Late-Stage Amination of Drug-Like Benzoic Acids: Access to Anilines and Drug Conjugates through Directed Iridium-Catalyzed C-H Activation
- PMID: 34672032
- PMCID: PMC9299223
- DOI: 10.1002/chem.202103510
Late-Stage Amination of Drug-Like Benzoic Acids: Access to Anilines and Drug Conjugates through Directed Iridium-Catalyzed C-H Activation
Abstract
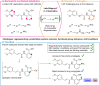
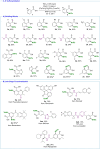
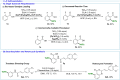
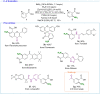
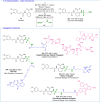
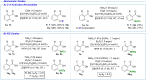
The functionalization of C-H bonds, ubiquitous in drugs and drug-like molecules, represents an important synthetic strategy with the potential to streamline the drug-discovery process. Late-stage aromatic C-N bond-forming reactions are highly desirable, but despite their significance, accessing aminated analogues through direct and selective amination of C-H bonds remains a challenging goal. The method presented herein enables the amination of a wide array of benzoic acids with high selectivity. The robustness of the system is manifested by the large number of functional groups tolerated, which allowed the amination of a diverse array of marketed drugs and drug-like molecules. Furthermore, the introduction of a synthetic handle enabled expeditious access to targeted drug-delivery conjugates, PROTACs, and probes for chemical biology. This rapid access to valuable analogues, combined with operational simplicity and applicability to high-throughput experimentation has the potential to aid and considerably accelerate drug discovery.
Keywords: C−H activation; amination; conjugation; high-throughput experimentation; iridium.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

