Interferon-stimulated gene 15 pathway is a novel mediator of endothelial dysfunction and aneurysms development in angiotensin II infused mice through increased oxidative stress
- PMID: 34672341
- PMCID: PMC9799052
- DOI: 10.1093/cvr/cvab321
Interferon-stimulated gene 15 pathway is a novel mediator of endothelial dysfunction and aneurysms development in angiotensin II infused mice through increased oxidative stress
Abstract
Aims: Interferon-stimulated gene 15 (ISG15) encodes a ubiquitin-like protein that induces a reversible post-translational modification (ISGylation) and can also be secreted as a free form. ISG15 plays an essential role as host-defence response to microbial infection; however, its contribution to vascular damage associated with hypertension is unknown.
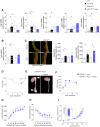
Methods and results: Bioinformatics identified ISG15 as a mediator of hypertension-associated vascular damage. ISG15 expression positively correlated with systolic and diastolic blood pressure and carotid intima-media thickness in human peripheral blood mononuclear cells. Consistently, Isg15 expression was enhanced in aorta from hypertension models and in angiotensin II (AngII)-treated vascular cells and macrophages. Proteomics revealed differential expression of proteins implicated in cardiovascular function, extracellular matrix and remodelling, and vascular redox state in aorta from AngII-infused ISG15-/- mice. Moreover, ISG15-/- mice were protected against AngII-induced hypertension, vascular stiffness, elastin remodelling, endothelial dysfunction, and expression of inflammatory and oxidative stress markers. Conversely, mice with excessive ISGylation (USP18C61A) show enhanced AngII-induced hypertension, vascular fibrosis, inflammation and reactive oxygen species (ROS) generation along with elastin breaks, aortic dilation, and rupture. Accordingly, human and murine abdominal aortic aneurysms showed augmented ISG15 expression. Mechanistically, ISG15 induces vascular ROS production, while antioxidant treatment prevented ISG15-induced endothelial dysfunction and vascular remodelling.
Conclusion: ISG15 is a novel mediator of vascular damage in hypertension through oxidative stress and inflammation.
Keywords: Endothelial dysfunction; ISG15; Inflammation; Oxidative stress; Vascular remodelling.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: none declared.
Figures






References
-
- Brandes RP. Endothelial dysfunction and hypertension. Hypertension 2014;64:924–928. - PubMed
-
- Laurent S, Boutouyrie P.. The structural factor of hypertension: large and small artery alterations. Circ Res 2015;116:1007–1021. - PubMed
-
- Sun X-N, Li C, Liu Y, Du L-J, Zeng M-R, Zheng X-J, Zhang W-C, Liu Y, Zhu M, Kong D, Zhou L, Lu L, Shen Z-X, Yi Y, Du L, Qin M, Liu X, Hua Z, Sun S, Yin H, Zhou B, Yu Y, Zhang Z, Duan S-Z.. T-cell mineralocorticoid receptor controls blood pressure by regulating interferon-gamma. Circ Res 2017;120:1584–1597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

