Effect of modified levonorgestrel-releasing intrauterine system in human adenomyosis with heavy menstrual bleeding
- PMID: 34672405
- PMCID: PMC9297895
- DOI: 10.1111/jog.15031
Effect of modified levonorgestrel-releasing intrauterine system in human adenomyosis with heavy menstrual bleeding
Abstract
Aim: The levonorgestrel-releasing intrauterine (LNG-IUS) system is an effective primary treatment for adenomyosis; however, it has high expulsion rates. We aimed to modify the system-allowing affixion to the myometrium-and evaluate the expulsion rate, effectiveness, and side effects in patients with adenomyosis and heavy menstrual bleeding.
Methods: This study included patients with adenomyosis and heavy menstrual bleeding who underwent implantation of: a modified LNG-IUS (experimental group, n = 47); and the original system after gonadotropin-releasing hormone agonist treatment (control group, n = 47), between January 2014 and April 2016.
Results: In the experimental group, two device expulsions occurred 12-18 months postimplantation. In the remaining 45 patients, the system was safely removed after the 60-month validity period, and no extrauterine device movement or infection occurred. In the control group, downward displacement and expulsion of the device occurred in eight (17%) patients within 60 months. The 5-year total expulsion rates were 4.3% and 17.0% in the experimental and control groups, respectively (p = 0.045). There were significant changes in the pretreatment severity of dysmenorrhea, menstrual volume, uterine volume (cm3 ), and hemoglobin level in each group compared with after 1 year (p < 0.01 in all groups). The severity of dysmenorrhea, menstrual volume, uterine volume, and hemoglobin level after 1 year were similar between the two groups (p > 0.05 in all groups).
Conclusions: Use of the modified LNG-IUS is a safe, cost-effective, and simple method for reducing the downward movement and expulsion rate in patients with adenomyosis and heavy menstrual bleeding.
Keywords: IUD expulsion; diffuse adenomyosis; heavy menstrual bleeding; hormone-releasing intrauterine devices; levonorgestrel.
© 2021 The Authors. Journal of Obstetrics and Gynaecology Research published by John Wiley & Sons Australia, Ltd on behalf of Japan Society of Obstetrics and Gynecology.
Conflict of interest statement
None declared.
Figures
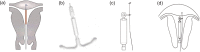

Similar articles
-
Effects of levonorgestrel-releasing intrauterine system and T380A intrauterine copper device on dysmenorrhea and days of bleeding in women with and without adenomyosis.Contraception. 2012 Nov;86(5):458-63. doi: 10.1016/j.contraception.2012.04.001. Epub 2012 Jul 24. Contraception. 2012. PMID: 22832203
-
Real-world outcomes of the levonorgestrel-releasing intrauterine system for heavy menstrual bleeding or dysmenorrhea in Japanese patients: A prospective observational study (J-MIRAI).Contraception. 2022 Dec;116:22-28. doi: 10.1016/j.contraception.2022.08.006. Epub 2022 Aug 31. Contraception. 2022. PMID: 36057322
-
Clinical experiences of the levonorgestrel-releasing intrauterine system in patients with large symptomatic adenomyosis.Taiwan J Obstet Gynecol. 2015 Aug;54(4):412-5. doi: 10.1016/j.tjog.2014.05.009. Taiwan J Obstet Gynecol. 2015. PMID: 26384061
-
The role of different LNG-IUS therapies in the management of adenomyosis: a systematic review and meta-analysis.Reprod Biol Endocrinol. 2025 Feb 13;23(1):23. doi: 10.1186/s12958-025-01349-4. Reprod Biol Endocrinol. 2025. PMID: 39948612 Free PMC article.
-
Levonorgestrel-releasing IUD as a method of contraception with therapeutic properties.Contraception. 1995 Nov;52(5):269-76. doi: 10.1016/0010-7824(95)00210-2. Contraception. 1995. PMID: 8585882 Review.
Cited by
-
Utility of the Levonorgestrel-Releasing Intrauterine System in the Treatment of Abnormal Uterine Bleeding and Dysmenorrhea: A Narrative Review.J Clin Med. 2022 Oct 1;11(19):5836. doi: 10.3390/jcm11195836. J Clin Med. 2022. PMID: 36233703 Free PMC article.
-
Effects of Shixiao Huoxue Decoction on pain, tumor necrosis factor-α, and interleukin-8 in patients with adenomyosis.Am J Transl Res. 2024 Feb 15;16(2):584-591. doi: 10.62347/VQVR4400. eCollection 2024. Am J Transl Res. 2024. PMID: 38463587 Free PMC article.
-
Adenomyosis and Abnormal Uterine Bleeding: Review of the Evidence.Biomolecules. 2024 May 23;14(6):616. doi: 10.3390/biom14060616. Biomolecules. 2024. PMID: 38927019 Free PMC article. Review.
-
From Diagnosis to Fertility: Optimizing Treatment of Adenomyosis for Reproductive Health.J Clin Med. 2024 Aug 21;13(16):4926. doi: 10.3390/jcm13164926. J Clin Med. 2024. PMID: 39201068 Free PMC article. Review.
References
-
- Li L, Leng JH, Jia SZ, et al. Analysis of unplanned taking‐out and expulsion of LNG‐IUS and related factors during the treatment of symptomatic adenomyosis (in Chinese). Chin J Pract Gynecol Obstet. 2016;11:1088–92.
-
- Youm J, Lee HJ, Kim SK, Kim H, Jee BC. Factors affecting the spontaneous expulsion of the levonorgestrel‐releasing intrauterine system. Int J Gynaecol Obstet. 2014;126:165–9. - PubMed
-
- Lockhat FB, Emembolu JO, Konje JC. The evaluation of the effectiveness of an intrauterine‐administered progestogen (levonorgestrel) in the symptomatic treatment of endometriosis and in the staging of the disease. Hum Reprod. 2004;19:179–84. - PubMed
-
- Park DS, Kim ML, Song T, et al. Clinical experiences of the levonorgestrel‐releasing intrauterine system in patients with large symptomatic adenomyosis. Taiwan J Obstet Gynecol. 2015;54:412–5. - PubMed
-
- Cooperative Group of Endometriosis, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association . Guideline for the diagnosis and treatment of endometriosis. Zhonghua Fu Chan Ke Za Zhi. 2015;50:161–9. (in Chinese). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

