2-Deoxy-D-glucose impedes T cell-induced apoptosis of keratinocytes in oral lichen planus
- PMID: 34672419
- PMCID: PMC8572795
- DOI: 10.1111/jcmm.16964
2-Deoxy-D-glucose impedes T cell-induced apoptosis of keratinocytes in oral lichen planus
Abstract
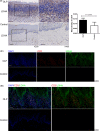
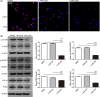
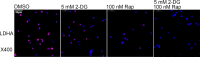
Oral lichen planus (OLP) is a T cell-mediated immunoinflammatory disease. Glycolysis plays an essential role in T-cell immune responses. Blocking glycolytic pathway in activated T cells represents a therapeutic strategy for restraint of immunologic process in autoimmune disorders. 2-Deoxy-D-glucose (2-DG) has been widely used to probe into glycolysis in immune cells. This study was aimed to explore the role of glycolysis inhibition by 2-DG on regulating immune responses of OLP-derived T cells. We observed that lactic dehydrogenase A (LDHA) expression was elevated in OLP lesions and local T cells. 2-DG inhibited the expression of LDHA, p-mTOR, Hif1α and PLD2 in T cells; meanwhile, it decreased proliferation and increased apoptosis of T cells. T cells treated by 2-DG showed lower LDHA expression and elevated apoptosis, resulting in a reduced apoptotic population of keratinocytes that were co-cultured with them, which was related to the decreased levels of IFN-γ in co-culture system. Rapamycin enhanced the effects of 2-DG on immune responses between T cells and keratinocytes. Thus, these findings indicated that OLP-derived T cells might be highly dependent upon high glycolysis for proliferation, and 2-DG treatment combined with rapamycin might be an option to alleviate T-cell responses, contributing to reducing apoptosis of keratinocytes.
Keywords: 2-deoxy-D-glucose; T cell; apoptosis; glycolysis; interferon-γ; keratinocyte; mammalian target of rapamycin; oral lichen planus.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declared no conflict of interest.
Figures




Similar articles
-
The mTOR-glycolytic pathway promotes T-cell immunobiology in oral lichen planus.Immunobiology. 2020 May;225(3):151933. doi: 10.1016/j.imbio.2020.151933. Epub 2020 Mar 9. Immunobiology. 2020. PMID: 32201095
-
Aberrant IGF1-PI3K/AKT/MTOR signaling pathway regulates the local immunity of oral lichen planus.Immunobiology. 2019 May;224(3):455-461. doi: 10.1016/j.imbio.2019.01.004. Epub 2019 Feb 10. Immunobiology. 2019. PMID: 30773287
-
HIF1α/PLD2 axis linked to glycolysis induces T-cell immunity in oral lichen planus.Biochim Biophys Acta Gen Subj. 2020 Jul;1864(7):129602. doi: 10.1016/j.bbagen.2020.129602. Epub 2020 Mar 20. Biochim Biophys Acta Gen Subj. 2020. PMID: 32205175
-
Pathogenesis of oral lichen planus--a review.J Oral Pathol Med. 2010 Nov;39(10):729-34. doi: 10.1111/j.1600-0714.2010.00946.x. Epub 2010 Oct 4. J Oral Pathol Med. 2010. PMID: 20923445 Review.
-
The pathogenesis of oral lichen planus.Crit Rev Oral Biol Med. 2002;13(4):350-65. doi: 10.1177/154411130201300405. Crit Rev Oral Biol Med. 2002. PMID: 12191961 Review.
Cited by
-
Effects of interleukin-23 on the activation of mucosal-associated invariant T cells from oral lichen planus.J Int Med Res. 2023 Jun;51(6):3000605231180039. doi: 10.1177/03000605231180039. J Int Med Res. 2023. PMID: 37340721 Free PMC article.
-
Association between oral lichen planus and Candida albicans infection: a systematic review and meta-analysis.Am J Transl Res. 2024 Aug 15;16(8):3462-3471. doi: 10.62347/WCDS1944. eCollection 2024. Am J Transl Res. 2024. PMID: 39262731 Free PMC article. Review.
-
Decrease of NAD+ Inhibits the Apoptosis of OLP T Cells via Inducing Mitochondrial Fission.J Inflamm Res. 2025 Jan 23;18:1091-1106. doi: 10.2147/JIR.S502273. eCollection 2025. J Inflamm Res. 2025. PMID: 39871961 Free PMC article.
-
Aberrant Activation of the STING-TBK1 Pathway in γδ T Cells Regulates Immune Responses in Oral Lichen Planus.Biomedicines. 2023 Mar 20;11(3):955. doi: 10.3390/biomedicines11030955. Biomedicines. 2023. PMID: 36979934 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

