Calsyntenin-3 interacts with the sodium-dependent vitamin C transporter-2 to regulate vitamin C uptake
- PMID: 34673103
- PMCID: PMC9842108
- DOI: 10.1016/j.ijbiomac.2021.10.058
Calsyntenin-3 interacts with the sodium-dependent vitamin C transporter-2 to regulate vitamin C uptake
Abstract
Ascorbic acid (AA) uptake in neurons occurs via a Na+-dependent carrier-mediated process mediated by the sodium-dependent vitamin C transporter-2 (SVCT2). Relatively little information is available concerning the network of interacting proteins that support human (h)SVCT2 trafficking and cell surface expression in neuronal cells. Here we identified the synaptogenic adhesion protein, calsyntenin-3 (CLSTN3) as an hSVCT2 interacting protein from yeast two-hybrid (Y2H) screening of a human adult brain cDNA library. This interaction was confirmed by co-immunoprecipitation, mammalian two-hybrid (M2H), and co-localization in human cell lines. Co-expression of hCLSTN3 with hSVCT2 in SH-SY5Y cells led to a marked increase in AA uptake. Reciprocally, siRNA targeting hCLSTN3 inhibited AA uptake. In the J20 mouse model of Alzheimer's disease (AD), mouse (m)SVCT2 and mCLSTN3 expression levels in hippocampus were decreased. Similarly, expression levels of hSVCT2 and hCLSTN3 were markedly decreased in hippocampal samples from AD patients. These findings establish CLSTN3 as a novel hSVCT2 interactor in neuronal cells with potential pathophysiological significance.
Keywords: Alzheimer's disease; CLSTN3; Interactor; SVCT2; Transport; Vitamin C.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest
The authors declare that there are no competing interests associated with the manuscript.
Figures


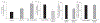

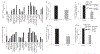
References
-
- Packer L, Fuchs J, Marcel Dekker Inc, New York, NY, USA, 1997.
-
- Dixit S, Bernardo A, Walker JM, Kennard JA, Kim GY, Kessler ES, Harrison FE, Vitamin C deficiency in the brain impairs cognition, increases amyloid accumulation and deposition, and oxidative stress in APP/PSEN1 and normally aging mice, ACS Chem. Neurosci. 6 (2015) 570–581, 10.1021/cn500308h. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

