Tractography methods and findings in brain tumors and traumatic brain injury
- PMID: 34673247
- PMCID: PMC8859988
- DOI: 10.1016/j.neuroimage.2021.118651
Tractography methods and findings in brain tumors and traumatic brain injury
Abstract
White matter fiber tracking using diffusion magnetic resonance imaging (dMRI) provides a noninvasive approach to map brain connections, but improving anatomical accuracy has been a significant challenge since the birth of tractography methods. Utilizing tractography in brain studies therefore requires understanding of its technical limitations to avoid shortcomings and pitfalls. This review explores tractography limitations and how different white matter pathways pose different challenges to fiber tracking methodologies. We summarize the pros and cons of commonly-used methods, aiming to inform how tractography and its related analysis may lead to questionable results. Extending these experiences, we review the clinical utilization of tractography in patients with brain tumors and traumatic brain injury, starting from tensor-based tractography to more advanced methods. We discuss current limitations and highlight novel approaches in the context of these two conditions to inform future tractography developments.
Keywords: Diffusion MRI; brain tumor; fiber tracking; glioma; tractography; traumatic brain injury.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest FY, AI, DBC, and AJG declare that this review was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Figures




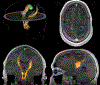

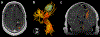
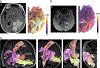

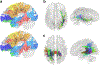
References
-
- Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL, 2001. Analysis of partial volume effects in diffusion-tensor MRI. Magn Reson Med 45, 770–780. - PubMed
-
- Alexopoulos G, Cikla U, El Tecle N, Kulkarni N, Pierson M, Mercier P, Kemp J, Coppens J, Mahmoud S, Sehi M, Bucholz R, Abdulrauf S, 2019. The Value of White Matter Tractography by Diffusion Tensor Imaging in Altering a Neurosurgeon’s Operative Plan. World Neurosurg 132, e305–e313. - PubMed
-
- Aliotta E, Moulin K, Ennis DB, 2018. Eddy current-nulled convex optimized diffusion encoding (EN-CODE) for distortion-free diffusion tensor imaging with short echo times. Magn Reson Med 79, 663–672. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

