VEGFR2 activity on myeloid cells mediates immune suppression in the tumor microenvironment
- PMID: 34673569
- PMCID: PMC8675197
- DOI: 10.1172/jci.insight.150735
VEGFR2 activity on myeloid cells mediates immune suppression in the tumor microenvironment
Abstract
Angiogenesis, a hallmark of cancer, is induced by vascular endothelial growth factor-A (hereafter VEGF). As a result, anti-VEGF therapy is commonly used for cancer treatment. Recent studies have found that VEGF expression is also associated with immune suppression in patients with cancer. This connection has been investigated in preclinical and clinical studies by evaluating the therapeutic effect of combining antiangiogenic reagents with immune therapy. However, the mechanisms of how anti-VEGF strategies enhance immune therapy are not fully understood. We and others have shown selective elevation of VEGFR2 expression on tumor-associated myeloid cells in tumor-bearing animals. Here, we investigated the function of VEGFR2+ myeloid cells in regulating tumor immunity and found VEGF induced an immunosuppressive phenotype in VEGFR2+ myeloid cells, including directly upregulating the expression of programmed cell death 1 ligand 1. Moreover, we found that VEGF blockade inhibited the immunosuppressive phenotype of VEGFR2+ myeloid cells, increased T cell activation, and enhanced the efficacy of immune checkpoint blockade. This study highlights the function of VEGFR2 on myeloid cells and provides mechanistic insight on how VEGF inhibition potentiates immune checkpoint blockade.
Keywords: Cancer immunotherapy; Immunology; Macrophages; Oncology.
Conflict of interest statement
Figures
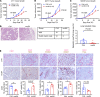



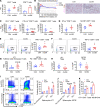
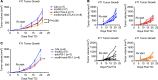
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

