Melanocortin 4 receptor stimulation prevents antidepressant-associated weight gain in mice caused by long-term fluoxetine exposure
- PMID: 34673574
- PMCID: PMC8670849
- DOI: 10.1172/JCI151976
Melanocortin 4 receptor stimulation prevents antidepressant-associated weight gain in mice caused by long-term fluoxetine exposure
Abstract
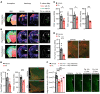
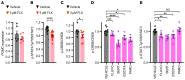
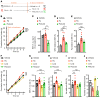
Contrasting with the predicted anorexigenic effect of increasing brain serotonin signaling, long-term use of selective serotonin reuptake inhibitor (SSRI) antidepressants correlates with body weight (BW) gain. This adverse outcome increases the risk of transitioning to obesity and interferes with treatment compliance. Here, we show that orally administered fluoxetine (Flx), a widely prescribed SSRI, increased BW by enhancing food intake in healthy mice at 2 different time points and through 2 distinct mechanisms. Within hours, Flx decreased the activity of a subset of brainstem serotonergic neurons by triggering autoinhibitory signaling through 5-hydroxytryptamine receptor 1a (Htr1a). Following a longer treatment period, Flx blunted 5-hydroxytryptamine receptor 2c (Htr2c) expression and signaling, decreased the phosphorylation of cAMP response element-binding protein (CREB) and STAT3, and dampened the production of pro-opiomelanocortin (POMC, the precursor of α-melanocyte stimulating hormone [α-MSH]) in hypothalamic neurons, thereby increasing food intake. Accordingly, exogenous stimulation of the melanocortin 4 receptor (Mc4r) by cotreating mice with Flx and lipocalin 2, an anorexigenic hormone signaling through this receptor, normalized feeding and BW. Flx and other SSRIs also inhibited CREB and STAT3 phosphorylation in a human neuronal cell line, suggesting that these noncanonical effects could also occur in individuals treated long term with SSRIs. By defining the molecular basis of long-term SSRI-associated weight gain, we propose a therapeutic strategy to counter this effect.
Keywords: Depression; Metabolism; Pharmacology; Psychiatric diseases; Therapeutics.
Conflict of interest statement
Figures





References
-
- Kane SP. https://clincalc.com/DrugStats/Drugs/Fluoxetine ClinCalc DrugStats Database, Version 21.1; 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

