Mycobacterium porcinum Skin and Soft Tissue Infections After Vaccinations - Indiana, Kentucky, and Ohio, September 2018-February 2019
- PMID: 34673748
- PMCID: PMC9361840
- DOI: 10.15585/mmwr.mm7042a3
Mycobacterium porcinum Skin and Soft Tissue Infections After Vaccinations - Indiana, Kentucky, and Ohio, September 2018-February 2019
Erratum in
-
Erratum: Vol. 70, No. 42.MMWR Morb Mortal Wkly Rep. 2021 Nov 5;70(44):1560. doi: 10.15585/mmwr.mm7044a3. MMWR Morb Mortal Wkly Rep. 2021. PMID: 34735424 Free PMC article. No abstract available.
Abstract
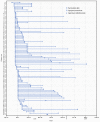
During December 2018-February 2019, a multistate investigation identified 101 patients with vaccination-associated adverse events among an estimated 940 persons in Kentucky, Indiana, and Ohio who had received influenza; hepatitis A; pneumococcal; or tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccines at the workplace during September 11-November 28, 2018. These vaccines had been administered by staff members of a third-party health care company contracted by 24 businesses. Company A provided multiple vaccine types during workplace vaccination events across 54 locations in these adjoining states. Injection-site wound isolates from patients yielded Mycobacterium porcinum, a nontuberculous mycobacteria (NTM) species in the Mycobacterium fortuitum group; subtyping using pulsed-field gel electrophoresis of all 28 available isolates identified two closely related clusters. Site visits to company A and interviews with staff members identified inadequate hand hygiene, improper vaccine storage and handling, lack of appropriate medical record documentation, and lack of reporting to the Vaccine Adverse Event Reporting System (VAERS). Vaccination-associated adverse events can be prevented by training health care workers responsible for handling or administering vaccines in safe vaccine handling, administration, and storage practices, timely reporting of any suspected vaccination-associated adverse events to VAERS, and notifying public health authorities of any adverse event clusters.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures

References
-
- Chojnacky MJ, Miller WW, Ripple DC, Strouse GF. Thermal analysis of refrigeration systems used for vaccine storage. Gaithersburg, MD: US Department of Commerce, National Institute of Standards and Technology; 2009. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.398.4818&rep=r...
-
- Objio T, Morelli V, Trimble S. Storage and handling [Chapter 5]. In: Hamborsky J, Kroger A, Wolfe S, eds. Epidemiology and prevention of vaccine-preventable diseases. 13th ed. Washington D.C.: Public Health Foundation; 2015. https://www.cdc.gov/vaccines/pubs/pinkbook/vac-storage.html
-
- Council of State and Territorial Epidemiologists. Standardized case definition for extrapulmonary nontuberculous mycobacteria infections. Atlanta, GA: Council of State and Territorial Epidemiologists; 2017.https://cdn.ymaws.com/www.cste.org/resource/resmgr/2017PS/2017PSFinal/17...
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

