The Plasmodium NOT1-G paralogue is an essential regulator of sexual stage maturation and parasite transmission
- PMID: 34673764
- PMCID: PMC8562791
- DOI: 10.1371/journal.pbio.3001434
The Plasmodium NOT1-G paralogue is an essential regulator of sexual stage maturation and parasite transmission
Abstract
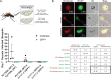
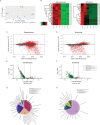
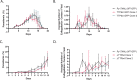
Productive transmission of malaria parasites hinges upon the execution of key transcriptional and posttranscriptional regulatory events. While much is now known about how specific transcription factors activate or repress sexual commitment programs, far less is known about the production of a preferred mRNA homeostasis following commitment and through the host-to-vector transmission event. Here, we show that in Plasmodium parasites, the NOT1 scaffold protein of the CAF1/CCR4/Not complex is duplicated, and one paralogue is dedicated for essential transmission functions. Moreover, this NOT1-G paralogue is central to the sex-specific functions previously associated with its interacting partners, as deletion of not1-g in Plasmodium yoelii leads to a comparable or complete arrest phenotype for both male and female parasites. We show that, consistent with its role in other eukaryotes, PyNOT1-G localizes to cytosolic puncta throughout much of the Plasmodium life cycle. PyNOT1-G is essential to both the complete maturation of male gametes and to the continued development of the fertilized zygote originating from female parasites. Comparative transcriptomics of wild-type and pynot1-g- parasites shows that loss of PyNOT1-G leads to transcript dysregulation preceding and during gametocytogenesis and shows that PyNOT1-G acts to preserve mRNAs that are critical to sexual and early mosquito stage development. Finally, we demonstrate that the tristetraprolin (TTP)-binding domain, which acts as the typical organization platform for RNA decay (TTP) and RNA preservation (ELAV/HuR) factors is dispensable for PyNOT1-G's essential blood stage functions but impacts host-to-vector transmission. Together, we conclude that a NOT1-G paralogue in Plasmodium fulfills the complex transmission requirements of both male and female parasites.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Genetic Characterization of Plasmodium Putative Pantothenate Kinase Genes Reveals Their Essential Role in Malaria Parasite Transmission to the Mosquito.Sci Rep. 2016 Sep 20;6:33518. doi: 10.1038/srep33518. Sci Rep. 2016. PMID: 27644319 Free PMC article.
-
Plasmodium falciparum PF10_0164 (ETRAMP10.3) is an essential parasitophorous vacuole and exported protein in blood stages.Eukaryot Cell. 2010 May;9(5):784-94. doi: 10.1128/EC.00336-09. Epub 2010 Mar 12. Eukaryot Cell. 2010. PMID: 20228203 Free PMC article.
-
An essential role for the Plasmodium Nek-2 Nima-related protein kinase in the sexual development of malaria parasites.J Biol Chem. 2009 Jul 31;284(31):20858-68. doi: 10.1074/jbc.M109.017988. Epub 2009 Jun 2. J Biol Chem. 2009. PMID: 19491095 Free PMC article.
-
Plasmodium falciparum gametocytes: still many secrets of a hidden life.Mol Microbiol. 2007 Oct;66(2):291-302. doi: 10.1111/j.1365-2958.2007.05904.x. Epub 2007 Sep 3. Mol Microbiol. 2007. PMID: 17784927 Review.
-
The s48/45 six-cysteine proteins: mediators of interaction throughout the Plasmodium life cycle.Int J Parasitol. 2017 Jun;47(7):409-423. doi: 10.1016/j.ijpara.2016.10.002. Epub 2016 Nov 27. Int J Parasitol. 2017. PMID: 27899328 Free PMC article. Review.
Cited by
-
Widespread release of translational repression across Plasmodium's host-to-vector transmission event.PLoS Pathog. 2025 Jan 8;21(1):e1012823. doi: 10.1371/journal.ppat.1012823. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39777415 Free PMC article.
-
Regulators of male and female sexual development are critical for the transmission of a malaria parasite.Cell Host Microbe. 2023 Feb 8;31(2):305-319.e10. doi: 10.1016/j.chom.2022.12.011. Epub 2023 Jan 11. Cell Host Microbe. 2023. PMID: 36634679 Free PMC article.
-
Supersaturation mutagenesis reveals adaptive rewiring of essential genes among malaria parasites.Science. 2025 Feb 7;387(6734):eadq7347. doi: 10.1126/science.adq7347. Epub 2025 Feb 7. Science. 2025. PMID: 39913589 Free PMC article.
-
Long-Read Genome Assembly and Gene Model Annotations for the Rodent Malaria Parasite Plasmodium yoelii 17XNL.bioRxiv [Preprint]. 2023 Jan 7:2023.01.06.523040. doi: 10.1101/2023.01.06.523040. bioRxiv. 2023. Update in: J Biol Chem. 2023 Jul;299(7):104871. doi: 10.1016/j.jbc.2023.104871. PMID: 36711553 Free PMC article. Updated. Preprint.
-
Gametogenesis in Plasmodium: Delving Deeper to Connect the Dots.Front Cell Infect Microbiol. 2022 Jun 15;12:877907. doi: 10.3389/fcimb.2022.877907. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35782151 Free PMC article. Review.
References
-
- World Health Organization. World Malaria Report 2020. Available from: https://www.who.int/publications/i/item/9789240015791.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

