Clinical characteristics and viral load dynamics of COVID-19 in a mildly or moderately symptomatic outpatient sample
- PMID: 34673816
- PMCID: PMC8530348
- DOI: 10.1371/journal.pone.0258970
Clinical characteristics and viral load dynamics of COVID-19 in a mildly or moderately symptomatic outpatient sample
Abstract
Background: Studies of outpatients with mild or moderate COVID-19 are uncommon. We studied: 1) association of symptoms with reverse transcriptase polymerase chain reaction (RT-PCR) test results; and 2) association of initial RT-PCR cycle threshold (Ct) in relation to duration of RT-PCR positivity in outpatients with mild or moderate COVID-19.
Methods: This was a cohort study of outpatients with confirmed COVID-19 and at least one symptom. Participants had repeat nasopharyngeal swabs and symptom checklists every 3-5 days until two consecutive RT-PCR tests were negative. RT-PCR tests were used to assess viral load. Antibody tests for COVID-19 were performed at 2 weeks, 4 weeks, and 8 weeks after symptom onset.
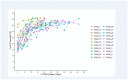
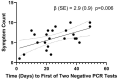
Results: Twenty-five patients (nine females) were enrolled, ranging in age from 19-58 (median age 28 years). All patients reported at least one symptom, with a median of six symptoms per patient. Symptoms persisted for 6-67 days (median duration 18 days). In all 25 patients, blood samples collected a median of 13 days after symptom onset were positive for SARS-CoV-2 antibodies in 15 (60%). After a median of 28 days following symptom onset, 23/23 patients with available samples tested positive for antibodies. The longest duration of positive RT-PCR test was 49 days from first positive PCR test (Mean = 27.4, SD = 12.5, Median = 24). Initial Ct was significantly associated with longer duration (β = -1.3, SE = 0.3, p<0.01 per 1 cycle higher) of RT-PCR positivity.
Conclusions: In mildly or moderately ill COVID-19 outpatients, RT-PCT tests remained positive for as long as 49 days and test positivity and symptom duration correlated with initial viral load.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Dynamics of viral load and anti-SARS-CoV-2 antibodies in patients with positive RT-PCR results after recovery from COVID-19.Korean J Intern Med. 2021 Jan;36(1):11-14. doi: 10.3904/kjim.2020.325. Epub 2020 Nov 25. Korean J Intern Med. 2021. PMID: 32972123 Free PMC article.
-
SARS-CoV-2 viral load dynamics and real-time RT-PCR cycle threshold interpretation in symptomatic non-hospitalised individuals in New Zealand: a multicentre cross sectional observational study.Pathology. 2021 Jun;53(4):530-535. doi: 10.1016/j.pathol.2021.01.007. Epub 2021 Mar 20. Pathology. 2021. PMID: 33838922 Free PMC article.
-
Relationship between viral load, infection-to-delivery interval and mother-to-child transfer of anti-SARS-CoV-2 antibodies.Ultrasound Obstet Gynecol. 2021 Jun;57(6):974-978. doi: 10.1002/uog.23639. Ultrasound Obstet Gynecol. 2021. PMID: 33798280 Free PMC article.
-
SARS-CoV-2 is associated with high viral loads in asymptomatic and recently symptomatic healthcare workers.PLoS One. 2021 Mar 18;16(3):e0248347. doi: 10.1371/journal.pone.0248347. eCollection 2021. PLoS One. 2021. PMID: 33735264 Free PMC article.
-
SARS-CoV-2 RT-PCR profile in 298 Indian COVID-19 patients: a retrospective observational study.Pathog Dis. 2021 Jan 9;79(1):ftaa064. doi: 10.1093/femspd/ftaa064. Pathog Dis. 2021. PMID: 33053181 Free PMC article.
Cited by
-
Fatal SARS-CoV-2 Reactivation After Allogeneic Hematopoietic Stem Cell Transplantation for Severe Aplastic Anemia.Cureus. 2025 Jul 1;17(7):e87084. doi: 10.7759/cureus.87084. eCollection 2025 Jul. Cureus. 2025. PMID: 40747163 Free PMC article.
-
COVID-19 symptom severity and duration among outpatients, July 2021-May 2023: The PROTECT observational study.PLoS One. 2025 Feb 21;20(2):e0314518. doi: 10.1371/journal.pone.0314518. eCollection 2025. PLoS One. 2025. PMID: 39982872 Free PMC article.
-
Viral dynamics and factors associated with duration of COVID-19 positivity: evidence from the first-three epidemiological waves in Cameroon.BMC Infect Dis. 2025 May 5;25(1):660. doi: 10.1186/s12879-025-11048-5. BMC Infect Dis. 2025. PMID: 40325359 Free PMC article.
-
Evaluation of symptomatology and viral load among residents and healthcare staff in long-term care facilities: A coronavirus disease 2019 retrospective case-cohort study.PLoS One. 2022 Nov 3;17(11):e0276796. doi: 10.1371/journal.pone.0276796. eCollection 2022. PLoS One. 2022. PMID: 36327239 Free PMC article.
-
Cycle Threshold Values as Indication of Increasing SARS-CoV-2 New Variants, England, 2020-2022.Emerg Infect Dis. 2023 Oct;29(10):2024-2031. doi: 10.3201/eid2910.230030. Epub 2023 Sep 7. Emerg Infect Dis. 2023. PMID: 37678158 Free PMC article.
References
-
- Centers for Disease Control and Prevention. Symptoms of Coronavirus 2021 [https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
-
- CITI Program. The CITI Program 2021 [https://about.citiprogram.org/en/homepage/.
-
- National Institutes of Health. Clinical Spectrum of SARS-CoV-2 Infection 2020 [March 21, 2021]. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/.
-
- Centers for Disease Control and Prevention. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel: Instructions for Use 2020 [CDC-006-00018, Revision 06:[https://www.fda.gov/media/134922/download.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

