Lapses in Care Among Patients Assigned to Ranibizumab for Proliferative Diabetic Retinopathy: A Post Hoc Analysis of a Randomized Clinical Trial
- PMID: 34673898
- PMCID: PMC8532036
- DOI: 10.1001/jamaophthalmol.2021.4103
Lapses in Care Among Patients Assigned to Ranibizumab for Proliferative Diabetic Retinopathy: A Post Hoc Analysis of a Randomized Clinical Trial
Abstract
Importance: The follow-up schedule for individuals with eyes treated with anti-vascular endothelial growth factor agents for proliferative diabetic retinopathy (PDR) requires that patients return frequently for monitoring and repeated treatment. The likelihood that a patient will comply should be a consideration in choosing a treatment approach.
Objective: To describe completion of scheduled examinations among participants assigned to intravitreous injections of ranibizumab for PDR in a multicenter randomized clinical trial.
Design, setting, and participants: This post hoc analysis evaluates data from a randomized clinical trial conducted at 55 US sites among 305 adults with proliferative diabetic retinopathy enrolled between February and December 2012. Both eyes were enrolled for 89 participants (1 eye to each study group), with a total of 394 study eyes. The final 2-year visit was completed in January 2015. Data were analyzed from April 2019 to July 2021.
Interventions: Ranibizumab injections for PDR or macular edema.
Main outcomes and measures: A long lapse in care of 8 or more weeks past a scheduled examination, dropout from follow-up, visual acuity at 5 years.
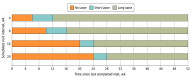
Results: Among 170 participants, the median age was 51 years, and 44.7% were female. Through 5 years of follow-up, 94 of 170 participants (55.3%) had 1 or more long lapse in care. Median time to the first long lapse was 210 weeks, and 69 of 94 participants (73.4%) returned for examination after the first long lapse. Fifty of 170 participants (29.4%) dropped out of follow-up by 5 years. Among the 120 participants who completed the 5-year examination, median change from baseline in visual acuity was -2 letters for participants who had 1 or more long lapse compared with +5 letters for those without a long lapse (P = .02). After multivariable adjustment, the odds ratio (95% CI) for baseline associations with 1 or more long lapse was 1.21 (1.03-1.43) for each 5-letter decrement in visual acuity score, 2.19 (1.09-4.38) for neovascularization of the disc and elsewhere, and 3.48 (1.38-8.78) for no prior laser treatment for diabetic macular edema.
Conclusions and relevance: Over 5 years, approximately half of the participants assigned to ranibizumab for PDR had a long lapse in care despite substantial effort by the DRCR Retina Network to facilitate timely completion of examinations. The likelihood of a long lapse in care during long-term follow-up needs to be considered when choosing treatment for PDR.
Trial registration: ClinicalTrials.gov Identifier: NCT01489189.
Conflict of interest statement
Figures

References
-
- Gross JG, Glassman AR, Jampol LM, et al. ; Writing Committee for the Diabetic Retinopathy Clinical Research Network . Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146. doi:10.1001/jama.2015.15217 - DOI - PMC - PubMed
-
- Gross JG, Glassman AR, Liu D, et al. ; Diabetic Retinopathy Clinical Research Network . Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138-1148. doi:10.1001/jamaophthalmol.2018.3255 - DOI - PMC - PubMed
-
- Sun JK, Glassman AR, Beaulieu WT, et al. ; Diabetic Retinopathy Clinical Research Network . Rationale and application of the Protocol S anti-vascular endothelial growth factor algorithm for proliferative diabetic retinopathy. Ophthalmology. 2019;126(1):87-95. doi:10.1016/j.ophtha.2018.08.001 - DOI - PMC - PubMed
-
- Sivaprasad S, Prevost AT, Vasconcelos JC, et al. ; CLARITY Study Group . Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193-2203. doi:10.1016/S0140-6736(17)31193-5 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

