Feeding Saccharomyces cerevisiae fermentation products lessens the severity of a viral-bacterial coinfection in preweaned calves
- PMID: 34673945
- PMCID: PMC8599294
- DOI: 10.1093/jas/skab300
Feeding Saccharomyces cerevisiae fermentation products lessens the severity of a viral-bacterial coinfection in preweaned calves
Abstract
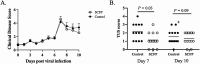
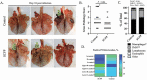
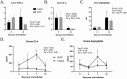
We have previously reported that supplementation with Saccharomyces cerevisiae fermentation products (SCFP) ameliorates clinical signs and lung pathology following experimental bovine respiratory syncytial virus (BRSV) infection in preweaned dairy calves. The objectives of this study were to determine the effect of SCFP supplementation on the metabolic and endocrine responses, and disease outcome of a viral-bacterial coinfection in preweaned calves. Twenty-seven, 1- to 2-d-old Holstein-Angus cross calves were enrolled in the study; one SCFP calf was removed from the trial during the pre-challenge phase due to complications from nephritis. Calves were assigned to two treatment groups: control or SCFP-treated, base milk replacer with 1 g/d SCFP (Smartcare, soluble formula) and calf starter top dressed with 5 g/d SCFP (NutriTek, insoluble formula). Calves were infected with BRSV on day 21, followed 6 d later by intratracheal inoculation with Pasteurella multocida (PM). Calves were euthanized on day 10 post-viral infection. Calves receiving SCFP had reduced thoracic ultrasonography scores on day 7 post-viral infection (P = 0.03) and a tendency toward reduced scores on day 10 post-viral infection (P = 0.09). Calves receiving SCFP also had less severe lung pathology scores at necropsy (P = 0.06). No differences between treatments were observed in lung viral loads (P = 0.48) or bacterial lung recovery (P = 0.34); however, there was a distinction in the lung location for PM recovery, with PM isolated more frequently from the cranial lobes in SCFP-treated calves, but more frequently from the caudal lobes of control calves. Calves treated with SCFP tended (P = 0.07) to have higher serum IL-6 concentrations following the coinfection. Calves treated with SCFP had lower concentrations of serum nonesterified fatty acids and beta-hydroxybutyric acid compared with controls following experimental challenge (P = 0.03 and P = 0.08, respectively), suggesting metabolic changes favoring growth and development. There were no differences between groups in gene expression of insulin receptor, insulin-like growth factor 1 (IGF-1), IGF-1 receptor (IGF-1R), growth hormone receptor, or haptoglobin in the liver. Results from this study suggest that supplementing with SCFP may moderate the impact of a respiratory viral-bacterial coinfection on preweaned calves through metabolic and immune modifications.
Keywords: Saccharomyces cerevisiae fermentation products; bovine respiratory disease; infection; innate immunity; metabolism; preweaned calves.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Society of Animal Science.
Figures



Similar articles
-
Supplementing a Saccharomyces cerevisiae fermentation product modulates innate immune function and ameliorates bovine respiratory syncytial virus infection in neonatal calves.J Anim Sci. 2020 Aug 1;98(8):skaa252. doi: 10.1093/jas/skaa252. J Anim Sci. 2020. PMID: 32780814 Free PMC article.
-
Feeding Saccharomyces cerevisiae fermentation postbiotic products alters immune function and the lung transcriptome of preweaning calves with an experimental viral-bacterial coinfection.J Dairy Sci. 2024 Apr;107(4):2253-2267. doi: 10.3168/jds.2023-23866. Epub 2023 Oct 6. J Dairy Sci. 2024. PMID: 37806633
-
Effects of feeding Saccharomyces cerevisiae fermentation products on the health of Holstein dairy calves following a lipopolysaccharide challenge.J Dairy Sci. 2022 Feb;105(2):1469-1479. doi: 10.3168/jds.2021-20341. Epub 2021 Nov 19. J Dairy Sci. 2022. PMID: 34802742
-
Influence of Saccharomyces cerevisiae fermentation products, SmartCare in milk replacer and Original XPC in calf starter, on the performance and health of preweaned Holstein calves challenged with Salmonella enterica serotype Typhimurium.J Dairy Sci. 2017 Sep;100(9):7154-7164. doi: 10.3168/jds.2016-12509. Epub 2017 Jul 19. J Dairy Sci. 2017. PMID: 28734601
-
Effects of Saccharomyces cerevisiae fermentation products on dairy calves: Ruminal fermentation, gastrointestinal morphology, and microbial community.J Dairy Sci. 2016 Jul;99(7):5401-5412. doi: 10.3168/jds.2015-10563. Epub 2016 May 4. J Dairy Sci. 2016. PMID: 27157569
Cited by
-
Impact of an Injectable Trace Mineral Supplement on the Immune Response and Outcome of Mannheimia haemolytica Infection in Feedlot Cattle.Biol Trace Elem Res. 2025 Mar;203(3):1475-1493. doi: 10.1007/s12011-024-04251-z. Epub 2024 Jun 10. Biol Trace Elem Res. 2025. PMID: 38853197 Free PMC article.
-
Effects of zinc supplementation and implant abscess on the immune system and growth performance of growing beef steers.Transl Anim Sci. 2024 May 2;8:txae075. doi: 10.1093/tas/txae075. eCollection 2024. Transl Anim Sci. 2024. PMID: 38764468 Free PMC article.
-
Postbiotics and Parabiotics in Veterinary Medicine: A Market Overview.Probiotics Antimicrob Proteins. 2025 Sep 1. doi: 10.1007/s12602-025-10734-9. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40889059 Review.
-
Fermentation Products Originated from Bacillus subtilis Promote Hepatic-Intestinal Health in Largemouth Bass, Micropterus salmoides.Biology (Basel). 2025 Jun 2;14(6):646. doi: 10.3390/biology14060646. Biology (Basel). 2025. PMID: 40563897 Free PMC article.
References
-
- Burdick Sanchez, N. C., Carroll J. A., Broadway P. R., Edrington T. S., Yoon I., and Belknap C. R.. . 2020. Some aspects of the acute phase immune response to a lipopolysaccharide (LPS) challenge are mitigated by supplementation with Saccharomyces cerevisiae fermentation product in weaned beef calves. Transl. Anim. Sci. 4:txaa156. doi:10.1093/tas/txaa156 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

