Cerebral derailment after myocardial infarct: mechanisms and effects of the signaling from the ischemic heart to brain
- PMID: 34674004
- PMCID: PMC8724191
- DOI: 10.1007/s00109-021-02154-3
Cerebral derailment after myocardial infarct: mechanisms and effects of the signaling from the ischemic heart to brain
Abstract
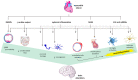
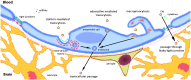
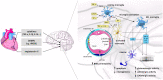
Myocardial infarction (MI) is the leading cause of death among ischemic heart diseases and is associated with several long-term cardiovascular complications, such as angina, re-infarction, arrhythmias, and heart failure. However, MI is frequently accompanied by non-cardiovascular multiple comorbidities, including brain disorders such as stroke, anxiety, depression, and cognitive impairment. Accumulating experimental and clinical evidence suggests a causal relationship between MI and stroke, but the precise underlying mechanisms have not yet been elucidated. Indeed, the risk of stroke remains a current challenge in patients with MI, in spite of the improvement of medical treatment among this patient population has reduced the risk of stroke. In this review, the effects of the signaling from the ischemic heart to the brain, such as neuroinflammation, neuronal apoptosis, and neurogenesis, and the possible actors mediating these effects, such as systemic inflammation, immunoresponse, extracellular vesicles, and microRNAs, are discussed.
Keywords: EVs; Myocardial infarct; Neuroinflammation; Stroke; miRNAs.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J (2017) Brain–heart interaction. Circ Res [Internet]. 121:451–68. Available from: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.117.311170 - DOI - PMC - PubMed
-
- Thackeray JT, Hupe HC, Wang Y, Bankstahl JP, Berding G, Ross TL et al (2018) Myocardial inflammation predicts remodeling and neuroinflammation after myocardial infarction. J Am Coll Cardiol [Internet]. 71:263–75. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735109717416408 - PubMed
-
- Battaglini D, Robba C, Lopes da Silva A, dos Santos Samary C, Leme Silva P, Dal Pizzol F et al (2020) Brain–heart interaction after acute ischemic stroke. Crit Care [Internet]. 24:163. Available from: https://ccforum.biomedical.com/articles/10.1186/s13054-020-02885-8 - DOI - PMC - PubMed
-
- Natelson BH (1985) Neurocardiology. Arch Neurol [Internet]. 42:178. Available from: https://archneur.jamanetwork.com/article.aspx?doi=10.1001/archneur.1985.... - PubMed
-
- Kaplan A, Yabluchanskiy A, Ghali R, Altara R, Booz GW, Zouein FA (2018) Cerebral blood flow alteration following acute myocardial infarction in mice. Biosci Rep [Internet]. 38. Available from: https://portlandpress.com/bioscirep/article/doi/10.1042/BSR20180382/8870... - DOI - PMC - PubMed

