Surrogate test performance for SARS-CoV-2 neutralizing antibodies (nAbs) for convalescent plasma (CCP): How useful could they be?
- PMID: 34674284
- PMCID: PMC8661940
- DOI: 10.1111/trf.16714
Surrogate test performance for SARS-CoV-2 neutralizing antibodies (nAbs) for convalescent plasma (CCP): How useful could they be?
Abstract
Background: COVID-19 high-titer CCP selection is a concern, because neutralizing antibody (nAb) testing requires sophisticated labs and methods. Surrogate tests are an alternative for measuring nAb levels in plasma bags, including those that are pathogen-reduced.
Study design/methods: We studied a panel consisting of 191 samples from convalescent donors tested by nAb (CPE-VNT), obtained from 180 CCP donations (collection: March 20-January 21) and 11 negative controls, with a total of 80 and 111 serum and plasma samples (71 amotosalen/UV treated), with nAb titers ranging from negative to 10,240. Samples were blindly tested for several surrogates: one anti-RBD, two anti-spike, and four anti-nucleocapsid tests, either isolated or combined to improve their positive predictive values as predictors of the presence of high-titer nAbs, defined as those with titers ≥160.
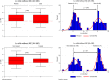
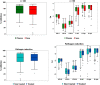
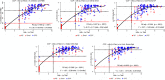
Results: Except for combined and anti-IgA/M tests, all isolated surrogate tests showed excellent performance for nAb detection: sensitivity (98.3%-100%), specificity (85.7%-100%), PPV (98.9%-100%), NPV (81.3%-100%), and AUC (0.93-0.96), with a variable decrease in sensitivity and considerably lower specificity when using FDA authorization and concomitant nAb titers ≥160. All surrogates had AUCs that were statistically different from CPE-VNT if nAb≥160, including when using combined, orthogonal approaches.
Conclusions: Surrogate tests (isolated or in combination) have an indirect good performance in detecting the presence of nAb, with lower sensitivity and specificity when high nAb titer samples are used, possibly accepting a considerable number of donors whose nAb titers are actually low, which should be evaluated by each laboratory responsible for CCP collection.
Keywords: COVID-19; SARS-CoV-2; coronavirus; convalescent plasma therapy; passive immune therapy; surrogate tests.
© 2021 The Authors. Transfusion published by Wiley Periodicals LLC on behalf of AABB.
Conflict of interest statement
The authors have disclosed no conflicts of interest.
Figures

Similar articles
-
A longitudinal study of convalescent plasma (CCP) donors and correlation of ABO group, initial neutralizing antibodies (nAb), and body mass index (BMI) with nAb and anti-nucleocapsid (NP) SARS-CoV-2 antibody kinetics: Proposals for better quality of CCP collections.Transfusion. 2021 May;61(5):1447-1460. doi: 10.1111/trf.16323. Epub 2021 Feb 19. Transfusion. 2021. PMID: 33604884 Free PMC article.
-
Selecting COVID-19 convalescent plasma for neutralizing antibody potency using a high-capacity SARS-CoV-2 antibody assay.Transfusion. 2021 Apr;61(4):1160-1170. doi: 10.1111/trf.16321. Epub 2021 Feb 18. Transfusion. 2021. PMID: 33554362 Free PMC article.
-
Simple prediction of COVID-19 convalescent plasma units with high levels of neutralization antibodies.Virol J. 2023 Mar 27;20(1):53. doi: 10.1186/s12985-023-02007-0. Virol J. 2023. PMID: 36973781 Free PMC article.
-
Clinical predictors of SARS-CoV-2 neutralizing antibody titers in COVID-19 convalescents: Implications for convalescent plasma donor recruitment.Eur J Haematol. 2021 Jul;107(1):24-28. doi: 10.1111/ejh.13630. Epub 2021 Apr 20. Eur J Haematol. 2021. PMID: 33780551 Free PMC article. Review.
-
A review of COVID-19 convalescent plasma use in COVID-19 with focus on proof of efficacy.Immunol Res. 2021 Feb;69(1):18-25. doi: 10.1007/s12026-020-09169-x. Epub 2021 Jan 25. Immunol Res. 2021. PMID: 33492637 Free PMC article. Review.
Cited by
-
The role of convalescent plasma and hyperimmune immunoglobulins in the COVID-19 pandemic, including implications for future preparedness.Front Immunol. 2024 Sep 9;15:1448720. doi: 10.3389/fimmu.2024.1448720. eCollection 2024. Front Immunol. 2024. PMID: 39315108 Free PMC article. Review.
-
Immunogenicity and safety of an intradermal fractional third dose of ChAdOx1 nCoV-19/AZD1222 vaccine compared with those of a standard intramuscular third dose in volunteers who previously received two doses of CoronaVac: A randomized controlled trial.Vaccine. 2022 Mar 15;40(12):1761-1767. doi: 10.1016/j.vaccine.2022.02.019. Epub 2022 Feb 21. Vaccine. 2022. PMID: 35210118 Free PMC article. Clinical Trial.
-
Distinct receptor binding domain IgG thresholds predict protective host immunity across SARS-CoV-2 variants and time.Nat Commun. 2023 Nov 2;14(1):7015. doi: 10.1038/s41467-023-42717-1. Nat Commun. 2023. PMID: 37919289 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

