Hypervalent Iodine-Mediated Late-Stage Peptide and Protein Functionalization
- PMID: 34674359
- PMCID: PMC9299824
- DOI: 10.1002/anie.202112287
Hypervalent Iodine-Mediated Late-Stage Peptide and Protein Functionalization
Abstract
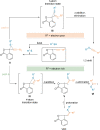
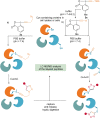
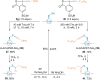
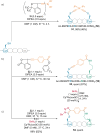
Hypervalent iodine compounds are powerful reagents for the development of novel transformations. As they exhibit low toxicity, high functional group tolerance, and stability in biocompatible media, they have been used for the functionalization of biomolecules. Herein, we report recent advances up to June 2021 in peptide and protein modification using hypervalent iodine reagents. Their use as group transfer or oxidizing reagents is discussed in this Minireview, including methods targeting polar, aromatic, or aliphatic amino acids and peptide termini.
Keywords: bioconjugation; hypervalent iodine; late-stage functionalization; peptides; proteins.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
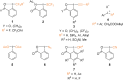
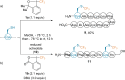

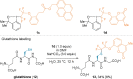
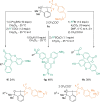
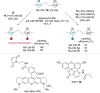
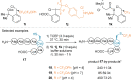
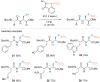
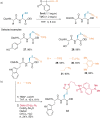



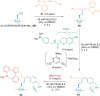
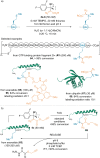
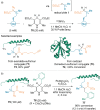

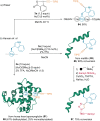
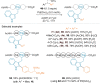

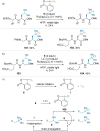
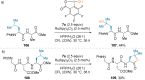
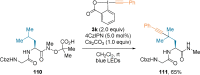

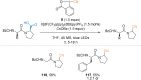

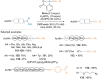
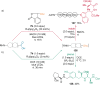
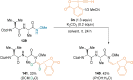
References
-
- None
-
- Leader B., Baca Q. J., Golan D. E., Nat. Rev. Drug Discovery 2008, 7, 21–39; - PubMed
-
- Carter P. J., Exp. Cell Res. 2011, 317, 1261–1269; - PubMed
-
- Kaspar A. A., Reichert J. M., Drug Discovery Today 2013, 18, 807–817; - PubMed
-
- Fosgerau K., Hoffman T., Drug Discovery Today 2015, 20, 122–128; - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

