The Triple-Tracer strategy against Metastatic PrOstate cancer (3TMPO) study protocol
- PMID: 34674367
- PMCID: PMC9546087
- DOI: 10.1111/bju.15621
The Triple-Tracer strategy against Metastatic PrOstate cancer (3TMPO) study protocol
Abstract
Objective: To determine the prevalence of intra-patient inter-metastatic heterogeneity based on positron emission tomography (PET)/computed tomography (CT) in patients with metastatic castration-resistant prostate cancer (mCRPC) and to determine the prevalence of neuroendocrine disease in these patients and their eligibility for radioligand therapies (RLTs).
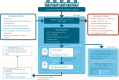
Patients and methods: This multicentre observational prospective clinical study will include 100 patients with mCRPC from five Canadian academic centres. Patients with radiological or biochemical progression and harbouring at least three metastases by conventional imaging will be accrued. Intra-patient inter-metastatic heterogeneity will be determined with triple-tracer imaging using fluorine-18 fluorodeoxyglucose (18 F-FDG), gallium-68-(68 Ga)-prostate-specific membrane antigen (PSMA)-617 and 68 Ga-DOTATATE, which are a glucose analogue, a PSMA receptor ligand and a somatostatin receptor ligand, respectively. The 68 Ga-PSMA-617 and 18 F-FDG PET/CT scans will be performed first. If at least one PSMA-negative/FDG-positive lesion is observed, an additional PET/CT scan with 68 Ga-DOTATATE will be performed. The tracer uptake of individual lesions will be assessed for each PET tracer and patients with lesions presenting discordant uptake profiles will be considered as having inter-metastatic heterogeneous disease and may be offered a biopsy.
Expected results: The proposed triple-tracer approach will allow whole-body mCRPC characterisation, investigating the inter-metastatic heterogeneity in order to better understand the phenotypic plasticity of prostate cancer, including the neuroendocrine transdifferentiation that occurs during mCRPC progression. Based on 68 Ga-PSMA-617 or 68 Ga-DOTATATE PET positivity, the potential eligibility of patients for PSMA and DOTATATE-based RLT will be assessed. Non-invasive whole-body determination of mCRPC heterogeneity and transdifferentiation is highly innovative and might establish the basis for new therapeutic strategies. Comparison of molecular imaging findings with biopsies will also link metastasis biology to radiomic features.
Conclusion: This study will add novel, biologically relevant dimensions to molecular imaging: the non-invasive detection of inter-metastatic heterogeneity and transdifferentiation to neuroendocrine prostate cancer by using a multi-tracer PET/CT strategy to further personalise the care of patients with mCRPC.
Keywords: #PCSM; #ProstateCancer; #uroonc; 18F-FDG; 68Ga-DOTATATE (Octreotate); 68Ga-PSMA-617; intra-patient inter-metastasis heterogeneity; metastatic castration-resistant prostate cancer; neuroendocrine differentiation; positron emission tomography/computed tomography.
© 2021 The Authors. BJU International published by John Wiley & Sons Ltd on behalf of BJU International.
Conflict of interest statement
The authors declare having received financial support of Merck Canada Inc. and of the Fonds de Recherche du Québec – Santé, as well as from the Cancer Research Society through the Oncopole organisation for the conduct of this study. The authors also acknowledge the contribution of Hermes Medical Solutions Canada for providing free infrastructure for imaging transfer. Dr Pouliot has received consultant honoraria or grants from Sanofi, Genzyme, Amgen, Astellas, Bayer and Janssen; he is a consultant/advisory board member of Sanofi, Ferring, Merck, Tersera, Amgen, Abbvie, Bayer, Astellas, Janssen, Genzyme and Eisai. Dr Kassouf received honorarium for Advisory Boards from the following companies: Ferring, Merck, Roche, BMS, EMD Serono/Pfizer, Sesen Bio, Janssen. Dr Probst is an Advisory Board member of Bayer. He has received consultant honoraria (speaking fees) from Bayer, Abbvie, Astellas, AAA, Isologics, Lantheus, Point Biopharma. Dr Anidjar has received grants as principal or co‐investigator from AngioDynamics Inc., Aragon Pharmaceuticals, Inc., Janssen Research and Development, Movember Foundation and Canadian Urology Research Consortium. The other authors have no potential conflicts of interest to declare.
Figures
References
-
- Schaeffer E, Srinivas S, Antonarakis ES et al. NCCN guidelines insights: prostate cancer, version 1.2021. J Natl Compr Canc Netw 2021; 19: 134–43 - PubMed
-
- Davies AH, Beltran H, Zoubeidi A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat Rev Urol 2018; 15: 271–86 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

