The IDeaS initiative: pilot study to assess the impact of rare diseases on patients and healthcare systems
- PMID: 34674728
- PMCID: PMC8532301
- DOI: 10.1186/s13023-021-02061-3
The IDeaS initiative: pilot study to assess the impact of rare diseases on patients and healthcare systems
Abstract
Background: Rare diseases (RD) are a diverse collection of more than 7-10,000 different disorders, most of which affect a small number of people per disease. Because of their rarity and fragmentation of patients across thousands of different disorders, the medical needs of RD patients are not well recognized or quantified in healthcare systems (HCS).
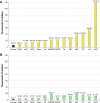
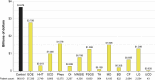
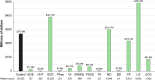
Methodology: We performed a pilot IDeaS study, where we attempted to quantify the number of RD patients and the direct medical costs of 14 representative RD within 4 different HCS databases and performed a preliminary analysis of the diagnostic journey for selected RD patients.
Results: The overall findings were notable for: (1) RD patients are difficult to quantify in HCS using ICD coding search criteria, which likely results in under-counting and under-estimation of their true impact to HCS; (2) per patient direct medical costs of RD are high, estimated to be around three-fivefold higher than age-matched controls; and (3) preliminary evidence shows that diagnostic journeys are likely prolonged in many patients, and may result in progressive, irreversible, and costly complications of their disease CONCLUSIONS: The results of this small pilot suggest that RD have high medical burdens to patients and HCS, and collectively represent a major impact to the public health. Machine-learning strategies applied to HCS databases and medical records using sentinel disease and patient characteristics may hold promise for faster and more accurate diagnosis for many RD patients and should be explored to help address the high unmet medical needs of RD patients.
Keywords: Diagnosis; Health care costs; Rare diseases; Utilization.
© 2021. The Author(s).
Conflict of interest statement
AT, AP, CC, JR, BL, DP, CH, DN, CHC, EG, HD, RN, PR and OS declare that they have no competing interests relevant to this manuscript. MH declares that she is the co-founder of Pryzm Health.
Figures



References
-
- Institute of Medicine (IOM). Chapter 2. Profile of rare diseases. IOM (US) committee on accelerating rare diseases research and orphan product development. In: Field MJ, Boat TF (eds). National Academies Press (US), Washington, DC; 2010. 10.17226/12953.
-
- NCOD (National Commission on Orphan Diseases). Report of the national commission on orphan diseases. Public Health Service, U.S. Department of Health and Human Services, Rockville, MD; 1989.
-
- NIH NCATS. Genetics and rare diseases information center. FAQs about rare diseases. https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-dise.... Accessed 14 April 2021.
-
- Haendel M, Vasilevsky N, Unni D, Bologa C, Harris N, Rehm H, Hamosh A, Baynam G, Groza T, McMurry J, Dawkins J, Rath A, Thaxon C, Bocci G, Joachimiak MP, Kohler S, Robinson PN, Mungall C, Oprea RI. How many rare diseases are there? Nat Rev Drug Discov. 2020;19(2):77–78. doi: 10.1038/d41573-019-00180-y. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

