Red blood cells and their releasates compromise bone marrow-derived human mesenchymal stem/stromal cell survival in vitro
- PMID: 34674751
- PMCID: PMC8529765
- DOI: 10.1186/s13287-021-02610-4
Red blood cells and their releasates compromise bone marrow-derived human mesenchymal stem/stromal cell survival in vitro
Abstract
Purpose: The use of bone marrow aspirate (BMA) and bone marrow aspirate concentrate (BMC) in the treatment of inflammatory orthopedic conditions has become a common practice. The therapeutic effect of BMA/BMC is thought to revolve primarily around the mesenchymal stem/stromal cell (MSC) population residing within the nucleated cell fraction. MSCs have the unique ability to respond to site of injury via the secretion of immunomodulating factors, resolving inflammation in diseased joints. Recently, the importance of hematocrit (HCT) in BMC has been debated, as the potential impact on MSC function is unknown. In the present study, we investigate MSC health over a short time-course following exposure to a range of HCT and red blood cell releasate (RBCrel) conditions.
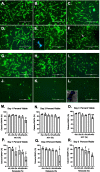
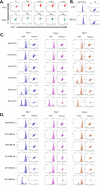
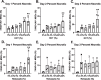
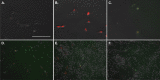
Methods: Bone marrow-derived human MSCs in early passage were grown under conditions of 0%, 2.5%, 5%, 10%, 20% and 40% HCT and RBCrel conditions for 3 days. At each day, the percentage of viable, apoptotic and necrotic MSCs was determined via flow cytometry. Relative viable MSC counts in each condition was determined to account for dynamic changes in overall MSC densities over the time-course. Statistical analysis was performed using a one-way ANOVA comparing test conditions to the control followed by a Dunnett's multiple comparison test.
Results: Significant reductions in viable MSCs concurrent with an increase in necrotic MSCs in high HCT and RBCrel conditions was observed within 24 h. At each successive timepoint, the percent and relative number of viable MSCs were reduced, becoming significant in multiple HCT and RBCrel conditions by Day 3. Necrosis appears to be the initial mode of MSC death following exposure to HCT and RBCrel, followed by apoptosis in surviving MSC fractions.
Conclusion: Various levels of HCT and RBCrel severely compromise MSC health within 3 days and HCT should be controlled in the preparation of BMC products. Further, HCT of BMCs should be routinely recorded and tracked with patient outcomes along with routine metrics (e.g. nucleated cell counts, fibroblast-colony forming units). Differences in HCT may account for the inconsistencies in the efficacy of BMC reported when treating orthopedic conditions.
Keywords: Apoptosis; Bone marrow aspirate concentrate; Hematocrit; Mesenchymal stem cells; Necrosis; Red blood cells; Viability.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
The Choice of Anticoagulant Influences the Characteristics of Bone Marrow Aspirate Concentrate and Mesenchymal Stem Cell Bioactivity In Vitro.Stem Cells Int. 2022 Jul 22;2022:8259888. doi: 10.1155/2022/8259888. eCollection 2022. Stem Cells Int. 2022. PMID: 35910535 Free PMC article.
-
"Cellularity as a predictive tool for mesenchymal stem cell concentration in bone marrow concentrates: Implications for regenerative medicine".Bone Rep. 2024 Nov 26;24:101820. doi: 10.1016/j.bonr.2024.101820. eCollection 2025 Mar. Bone Rep. 2024. PMID: 39691445 Free PMC article.
-
Single- Versus Multiple-Site Harvesting Techniques for Bone Marrow Concentrate: Evaluation of Aspirate Quality and Pain.Orthop J Sports Med. 2017 Aug 29;5(8):2325967117724398. doi: 10.1177/2325967117724398. eCollection 2017 Aug. Orthop J Sports Med. 2017. PMID: 28890905 Free PMC article.
-
Mesenchymal stromal cell and bone marrow concentrate therapies for musculoskeletal indications: a concise review of current literature.Mol Biol Rep. 2020 Jun;47(6):4789-4814. doi: 10.1007/s11033-020-05428-0. Epub 2020 May 25. Mol Biol Rep. 2020. PMID: 32451926 Free PMC article. Review.
-
Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells.Stem Cells Dev. 2012 Sep 20;21(14):2724-52. doi: 10.1089/scd.2011.0722. Epub 2012 May 9. Stem Cells Dev. 2012. PMID: 22468918 Review.
Cited by
-
The Choice of Anticoagulant Influences the Characteristics of Bone Marrow Aspirate Concentrate and Mesenchymal Stem Cell Bioactivity In Vitro.Stem Cells Int. 2022 Jul 22;2022:8259888. doi: 10.1155/2022/8259888. eCollection 2022. Stem Cells Int. 2022. PMID: 35910535 Free PMC article.
-
Optimization of Mesenchymal Stromal Cell (MSC) Manufacturing Processes for a Better Therapeutic Outcome.Front Immunol. 2022 Jun 9;13:918565. doi: 10.3389/fimmu.2022.918565. eCollection 2022. Front Immunol. 2022. PMID: 35812460 Free PMC article. Review.
-
Pharmacokinetic characteristics of mesenchymal stem cells in translational challenges.Signal Transduct Target Ther. 2024 Sep 13;9(1):242. doi: 10.1038/s41392-024-01936-8. Signal Transduct Target Ther. 2024. PMID: 39271680 Free PMC article. Review.
-
Side-to-side characterisation of cellular content, soluble factors and in vitro potential on chondrocytes for bone marrow aspirate concentrate and adipose-derived stromal vascular fraction.J Exp Orthop. 2025 May 12;12(2):e70254. doi: 10.1002/jeo2.70254. eCollection 2025 Apr. J Exp Orthop. 2025. PMID: 40357029 Free PMC article.
-
The Potential of Red Blood Cells in Regenerative Medicine: A Paradigm Shift in Cellular Therapy.Cells. 2025 May 29;14(11):797. doi: 10.3390/cells14110797. Cells. 2025. PMID: 40497973 Free PMC article. Review.
References
-
- Garbin LC, Olver CS. Platelet-rich products and their application to osteoarthritis. J Equine Vet Sci. 2020;86:102820. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

